
Navigation of tumor location and intersegmental planes utilizing mixed reality in video-assisted thoracic surgery for non-small cell lung cancer: a case report of right S2 segmentectomy
Highlight box
Key findings
• Mixed reality (MR) technology in lung cancer segmentectomy for precise tumor localization
What is known and what is new?
• Existing tumor marking methods can be invasive and less accurate
• MR technology provides non-invasive, effective tumor marking during segmentectomy
What is the implication, and what should change now?
• MR could revolutionize lung cancer surgeries
• Further research on MR’s practicality in thoracic surgeries is crucial.
Introduction
With the widespread use of computed tomography (CT), the number of small lung cancers detected has increased significantly. Recent clinical trials conducted in Japan and the United States have highlighted the potential benefits of sublobar resection, leading to a growing adoption of this technique (1-3).
One of the main challenges in sublobar resection is ensuring adequate tumor margins. Several tumor marking methods have been explored to address this issue, but some come with inherent risks. Among these methods, the indocyanine green (ICG) method has gained popularity in recent years. However, it too has limitations. Although ICG provides visual guidance, it falls short in establishing precise spatial relationships with the actual tumor, and, in certain cases, struggles with intersegmental delineation (4).
In recent years, mixed reality (MR), a technology that merges elements of virtual and augmented reality, has found applications in the realm of surgical treatment (5,6). The utilization of MR for tumor marking holds promise as a potential solution. It offers a non-invasive and relatively straightforward approach, potentially representing the next generation of treatments. This technology has the capacity to enable safe and reliable surgery by accurately pinpointing the location of the tumor and intersegmental boundaries. To the best of our knowledge, this report marks the first documented instance of segmentectomy for lung cancer utilizing MR. We present this case in accordance with the CARE reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-23-58/rc).
Case presentation
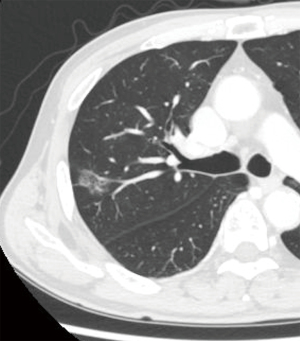
A man in his 70s presented with a pure ground-glass nodule (GGN) in the right upper lobe, detected during routine follow-up CT scans conducted at a local clinic for adrenal insufficiency. Over the course of 2 years, the pulmonary nodule grew to a size of 2.1 cm. A transbronchial lung biopsy confirmed the nodule as lung adenocarcinoma, prompting the decision to proceed with surgery. Patient medical history included diabetes mellitus. Chest CT identified a 2.1 cm × 1.3 cm pure GGN located peripherally in segment 2 (S2) of the right upper lobe (Figure 1). Positron emission tomography (PET)/CT revealed no abnormal uptake. The clinical stage was diagnosed as c-TisN0M0 stage 0, and a right S2 segmentectomy was planned using four-port video-assisted thoracic surgery (VATS). Based on the data from the JCOG1211 trial (3), we elected to perform a segmentectomy as the lesion was a pure GGN exceeding 2 cm.
A preoperative CT scan using a 1.25 mm slice thickness was performed to capture the entire lung field. Additionally, Synapse Vincent software (Fujifilm, Tokyo, Japan) was employed to construct a three-dimensional (3D) model of the lung, as previously reported (7,8). Individual components of the 3D lung model including the lung lobe, pulmonary artery, pulmonary vein, bronchus, tumor, and targeted lung segments for resection, were exported as “STL files”. Each file is exported separately. These STL files were then uploaded to Holoeyes XR (Holoeyes, Tokyo, Japan), a platform provided by Holoeyes Inc., where the 3D lung model was reconstructed online. The 3D lung model was then imported into HoloLens 2 (Microsoft Corporation, WA, USA), a head-mounted display, and its visualization was confirmed using HoloLens 2. During the surgery, either an assistant or external aid manipulated the HoloLens 2.
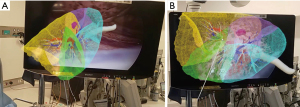
Following port placement, a VATS right S2 segmentectomy was planned. Intrathoracic visualization was achieved using a thoracoscopic camera, with an assistant equipped with a HoloLens 2 projected a 3D lung model onto the surgical field, creating a MR environment (Figure 2A). By aligning the lung landmarks from this model with those visible on the thoracoscopic monitor, congruence between the actual lung view and the 3D model was ensured, facilitating accurate identification of the tumor location and segmental delineation (Figure 2B).
Subsequently, a right S2 segmentectomy was performed via VATS. MR technology was utilized to mark the segmental boundaries on the lung surface, with the pleura labeled using soft coagulation (Figure 3A). After posterior mediastinal pleura dissection, the B2 bronchus was identified. From the interlobar region, the pulmonary artery A2 was dissected, and veins V2c and V2b, branching from the central vein, were ligated and resected while preserving V2a. After stapling the right B2, ICG was intravenously injected, and observation with an infrared thoracoscope (VISERA ELITE II, Olympus, Tokyo, Japan) during segmental delineation showed a faint depiction, which was not clear, possibly because of pulmonary emphysema (Figure 3B). Utilizing MR-guided delineation of the segmental boundary, the intersegmental planes were resected using staplers, completing S2 segmentectomy (Figure 3C). Frozen section analysis verified a secure margin of 1.9 cm. The drain was removed on the second postoperative day, and the patient showed favorable progress with no signs of recurrence. The pathological diagnosis revealed minimally invasive adenocarcinoma with a maximum tumor diameter of 2.1 cm and an invasive diameter of 0.2 cm. Staging was determined as pT1miN0M0 (stage IA1).

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In this case, we performed a right S2 segmentectomy using VATS for small-sized lung adenocarcinoma primarily characterized by radiologically pure GGN. Our surgical approach was notably enhanced by integrating MR technology to mark the intersegmental plane, facilitating precise tumor localization. Although attempts to delineate intersegmental planes using conventional intravenous ICG have been inconclusive, MR-assisted segmentectomy consistently provided adequate surgical margins. To the best of our knowledge, this represents the first reported instance of segmentectomy assisted by MR technology.
The integration of MR and thoracic surgery marks a groundbreaking development in thoracoscopic surgery (9-11). MR technology seamlessly combines virtual objects with the real world, enabling surgeons to interact with a dynamic real-time 3D lung model in the operating room, serving as a valuable visual guide. Notably, by creating a detailed 3D lung model using Synapse Vincent and superimposing it on a monitor showing the actual surgical field, the system enabled surgical navigation with unparalleled accuracy. Prior to these innovations, surgeons often relied on CT-guided marking, dye marking, or Endofinger methods for intraoperative tumor location as preoperative markers (12). Although these traditional methods are effective, they lack the dynamism and real-time performance offered by MR.
Several methods for tumor localization and intersegmental plane identification in thoracic surgery have been reported. Tumor identification in thoracic surgery has evolved through various stages of development; techniques such as CT-guided hook wire placement, dye and lipiodol injection, radial bronchoscopic ICG dispersing, coil or radiofrequency identification microchip implantation, and Virtual Assisted Lung Mapping have been considered highly effective methods (12-14). Nevertheless, each method has inherent challenges. Although these techniques ensure a certain degree of accuracy, they are invasive and often carry the risk of serious complications. Furthermore, despite meticulous implementation, the potential for misidentification remains, and in VATS, this challenge is magnified by the limited space in the thoracic cavity. Notably, the ease of palpation diminishes as the tumor size decreases, and detection typically occurs at an earlier stage. Although each method has distinct advantages, they also present unique challenges, highlighting the need for enhanced and more reliable tools.
Conversely, several methods exist for identifying the intersegmental plane (15). These include air-containing collapse, intravenous ICG, and hyperinflation methods. Of these, ICG is particularly useful for narrow thoracic spaces encountered during VATS. However, despite its usefulness in intersegmental delineation, the ICG technique sometimes fails to provide optimal delineation. In such cases, clear intersegmental boundaries were still not captured. This underscores the fact that even the most established intersegmental identification methods can encounter setbacks and reinforces the need for improved and more reliable approaches.
The exceptional advantage of the MR technology lies its unique capacity to bridge the virtual and real worlds. This allows the surgeon to predict the tumor location and intersegmental plane by adjusting the visual scale and matching the landmarks of the lung structures. This is particularly powerful when MR markings are made immediately after thoracoscopic surgery, especially when the lungs are in a minimally collapsed phase. Although innovation is promising, it often comes with challenges. One important challenge is the scaling and alignment of the 3D lung model. Overlaying a 3D lung model with collapsed lungs caused by isolated lung ventilation requires attention to both technical and anatomical aspects, as well as expertise and experience. Although it is difficult to accurately reflect the degree of pulmonary collapse in the model, it was addressed by assuming that collapse is centered at the pulmonary hilum and applying a uniform shrinkage to the entire lung. As the second limitation, the MR imaging technique may be compromised in the presence of severe adhesions, although feasible after detachment of the adhesions. To ensure safety, the ICG method was concurrently used for segmentectomy in this case and remained effective. However, as MR technology matures and is performed more seamlessly, its reliability increases, potentially reducing the problems currently encountered with MR. Furthermore, it is worth mentioning that MR can be applied to wedge resection as well.
Conclusions
In conclusion, this case serves as a significant milestone in technological innovations within thoracic surgery. It not only underscores the transformative potential of technologies such as MR but also highlights the necessity for ongoing research and adaptation. Through the use of MR, we successfully identified the tumor and delineated the intersegmental plane in VATS segmentectomy, ensuring adequate resection margins for the procedure. MR is anticipated to drive further advancements that will augment the intuitive decision-making of the surgeon, ultimately enhancing both the safety and effectiveness of surgical interventions.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Seshiru Nakazawa and Hitoshi Igai) for the series “Simulation and Navigation Techniques in VATS/RATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-23-58/rc
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-23-58/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-23-58/coif). The series “Simulation and Navigation Techniques in VATS/RATS” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Aokage K, Suzuki K, Saji H, et al. Segmentectomy for ground-glass-dominant lung cancer with a tumour diameter of 3 cm or less including ground-glass opacity (JCOG1211): a multicentre, single-arm, confirmatory, phase 3 trial. Lancet Respir Med 2023;11:540-9. [Crossref] [PubMed]
- Kasai Y, Tarumi S, Chang SS, et al. Clinical trial of new methods for identifying lung intersegmental borders using infrared thoracoscopy with indocyanine green: comparative analysis of 2- and 1-wavelength methods. Eur J Cardiothorac Surg 2013;44:1103-7. [Crossref] [PubMed]
- Kitagawa M, Sugimoto M, Haruta H, et al. Intraoperative holography navigation using a mixed-reality wearable computer during laparoscopic cholecystectomy. Surgery 2022;171:1006-13. [Crossref] [PubMed]
- Sadeghi AH, Maat APWM, Taverne YJHJ, et al. Virtual reality and artificial intelligence for 3-dimensional planning of lung segmentectomies. JTCVS Tech 2021;7:309-21. [Crossref] [PubMed]
- Ikeda N, Yoshimura A, Hagiwara M, et al. Three dimensional computed tomography lung modeling is useful in simulation and navigation of lung cancer surgery. Ann Thorac Cardiovasc Surg 2013;19:1-5. [Crossref] [PubMed]
- Kudo Y, Ikeda N. Benefits of lung modeling by high-quality three-dimensional computed tomography for thoracoscopic surgery. Video-assist Thorac Surg 2019;4:4. [Crossref]
- Fu R, Zhang C, Zhang T, et al. A three-dimensional printing navigational template combined with mixed reality technique for localizing pulmonary nodules. Interact Cardiovasc Thorac Surg 2021;32:552-9. [Crossref] [PubMed]
- Perkins SL, Krajancich B, Yang CJ, et al. A Patient-Specific Mixed-Reality Visualization Tool for Thoracic Surgical Planning. Ann Thorac Surg 2020;110:290-5. [Crossref] [PubMed]
- Vervoorn MT, Wulfse M, Van Doormaal TPC, et al. Mixed Reality in Modern Surgical and Interventional Practice: Narrative Review of the Literature. JMIR Serious Games 2023;11:e41297. [Crossref] [PubMed]
- Sato M. Precise sublobar lung resection for small pulmonary nodules: localization and beyond. Gen Thorac Cardiovasc Surg 2020;68:684-91. [Crossref] [PubMed]
- Sato T, Yutaka Y, Nakamura T, et al. First clinical application of radiofrequency identification (RFID) marking system-Precise localization of a small lung nodule. JTCVS Tech 2020;4:301-4. [Crossref] [PubMed]
- Sato M, Omasa M, Chen F, et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg 2014;147:1813-9. [Crossref] [PubMed]
- Onodera K, Suzuki J, Miyoshi T, et al. Comparison of various lung intersegmental plane identification methods. Gen Thorac Cardiovasc Surg 2023;71:90-7. [Crossref] [PubMed]
Cite this article as: Kudo Y, Omori T, Amemiya R, Ikeda N. Navigation of tumor location and intersegmental planes utilizing mixed reality in video-assisted thoracic surgery for non-small cell lung cancer: a case report of right S2 segmentectomy. Video-assist Thorac Surg 2024;9:13.


