
Efficacy of radiofrequency identification lung marking system in revision surgery: a case report
Highlight box
Key findings
• A minimally invasive procedure was performed using radiofrequency identification (RFID) lung marking system in a patient with a history of ipsilateral thoracic surgery and extensive adhesions in the thoracic cavity.
What is known and what is new?
• Various approaches have been devised for small lung cancer. The challenge was how to mark cases with emphysema and adhesions, as well as nodules located deep in the body.
• We describe our clinical experience with a new tumor identification method, the RFID lung marking system, which was useful for small lung cancer with adhesions.
What is the implication, and what should change now?
• It is a minimally invasive surgical technique that is expected to become more widely used in the future.
Introduction
Background
With recent improvements in computed tomography (CT) imaging, the frequency of small lung nodule detection has increased. In surgeries targeting small lung nodules, it is important to identify the location of the tumor and ensure a sufficient margin. Lung marking systems have been developed to localize and resect small nodules. Despite these advancements, fatal complications, such as air embolism, have been reported for CT-guided needle-mediated methods (1) and dye marking is not visible in centrally located tumors in patients with anthracotic lung or emphysema in virtual-assisted lung mapping (VAL-MAP) (2). In addition, effective marking methods for cases of adhesions are challenging. Here, we report a case of heterochronic lung cancer with extensive adhesions in the thoracic cavity that was successfully resected using a radiofrequency identification (RFID) lung marking system. We present this case in accordance with the CARE reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-23-59/rc).
Case presentation
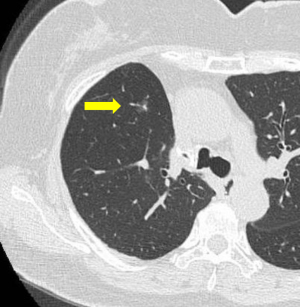
The patient was a 62-year-old woman. After thoracoscopic resection of multiple lung cancers in the right upper middle lobe, a small nodule appeared in the right lower lobe during outpatient follow-up. The tumor showed mild enlargement, and she requested surgery. The results of physiological and laboratory examinations were normal. She had a medical history of multiple right lung cancers and diabetes mellitus. The patient had no history of smoking. CT noted a 10 mm pure ground-glass nodule (GGN) at a relatively deep location in the right lower lobe (Figure 1). The nodule was located relatively deep in the lung, and the fact that it was a pure GGN also raised concerns about the difficulty in identifying the tumor and the insufficient depth margin. Furthermore, because the patient had undergone a thoracoscopic right upper middle lobectomy one year earlier, a wide area of adhesion was expected in the right thoracic cavity. Preoperative marking using the RFID lung marking system was recommended, and the integrated circuit (IC) tag was implanted two days before surgery.
Virtual bronchoscopy images and 3D images were created using the software Synapse Vincent®︎ (Fujifilm, Tokyo, Japan) and were used for preoperative IC tag placement.
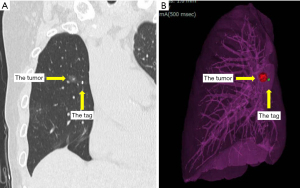
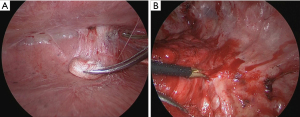
The bronchoscope was guided by a virtual endoscopic image and the IC tag was implanted near the tumor during fluoroscopy and cone-beam CT (Figure 2). The IC tag was placed in B8a bronchus, 7 mm mediastinal side from the tumor. After preoperative marking, CT was performed to create a 3D image for surgery; the 3D image was taken to confirm the marker location and target nodule (Figure 3). Surgery was performed via a three-port VATS with the patient under general anesthesia. The thoracic cavity showed full adhesions to all chest walls, diaphragm, mediastinum, and pericardium. Especially just under the wound used in the previous surgery, a strong adhesion was observed (Figure 4). First, all adhesions were detached, and palpation of the tumor was tried, however, the tumor was difficult to palpate and identify, as expected preoperatively, because it was a deeply located small nodule and had been affected by adhesion dissection. A surgical antenna (diameter: 10 mm) was used to detect RFID tags. Signal detection is achieved almost immediately. There were five different detection tones, depending on the distance from the tag. The resection line was designed by marking the position where the highest tone was detected. Wedge resection was performed by using an automatic suturing device. We used a surgical antenna to check each time for sufficiently deep margins while performing the resection (Video 1). The tag was found on the deep side of the tumor, providing a 15 mm resection margin. Pathological examination confirmed that the target nodule was a minimally invasive adenocarcinoma (invasive size: 2 mm). The patient was discharged without any post-operative complications eight days after the operation and was followed up for two years post-operatively without any recurrence.

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images/video. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Lobectomy has long been the standard treatment for early stage non-small cell lung cancer (NSCLC). However, the survival and clinical benefits of segmentectomy were examined in a recent randomized trial to determine whether segmental resection was non-inferior to lobectomy in patients with small peripheral NSCLC (3). A multicenter, noninferiority phase III trial was conducted, in which patients with NSCLC tumors ≤2 cm in diameter were randomized to undergo either sublobar resection or lobectomy after intraoperative confirmation of negative lymph node metastases. The trial demonstrated that sublobar resection was not inferior to lobectomy in terms of disease-free survival (4). Additionally, a single-arm study was conducted to evaluate the efficacy and safety of sublobar resection (wide wedge resections or segmentectomy) for ground-glass opacity-dominant peripheral lung cancer. The study demonstrated that sublobar resection with enough surgical margins provided adequate local control and recurrence-free survival for peripheral lesions of 2.0 cm or less with a consolidation tumor ratio of 0.25 or less. Macroscopic surgical margins must be at least 5 mm, and this criterion is substantiated by evaluating the distance between the tumor and the staple or incision line in the parenchyma. The median pathological surgical margin was 15 mm (range, 0–55 mm) (5).
It is expected that the results will be reflected in these findings and that the frequency of sublobar resection will increase. In surgeries aimed at addressing small lung nodules, precise identification of the tumor and assurance of adequately deep margins are of paramount importance. CT-guided hook-wire localization is widely used as a preoperative marking method for small lung nodules. Air embolisms have been reported as a severe complication. VAL-MAP is a useful method, but dye recognition can be difficult in patients with emphysema or silicosis (6). Both these methods are lung surface marking methods for the visceral pleura, and it was concerning that they lacked certainty in ensuring deep margins. VAL-MAP 2.0 is a combined multiple dye marks of conventional VAL-MAP with endobronchial microcoils to provide navigation for thoracoscopic deep lung resection (7). Many facilities use CT-guided cutaneous and pleural marking, marking the skin directly above the tumor under CT guidance before surgery and placing a pleural marker with dye on the parietal pleura directly below the region marked on the skin using a catheter needle (8). Various methods have been proposed and reported, including a combination of VAL-MAP, CT-guided percutaneous localization, and Lipiodol marking (9,10). Each marking method has its own strengths and limitations, and the selection depends on the preferences and practices of individual facilities. A summary of these methods is described (Table 1).
Table 1
| Methods | Marking method | Requirements | In clinical practice | Limitations | AEs |
|---|---|---|---|---|---|
| CT-guided hook wire localization | Hook wire | Cooperation of radiologist | Hook wire localization was performed by an interventional radiologist in the CT room just before transferring the patient to the operating room for surgery | AEs | Pneumothorax |
| Pre interventional CT scan | Wire dropout | Intrapulmonary hemorrhage | |||
| post interventional CT scan | Deep margin | Hook-wire dislodgement | |||
| Air embolism | |||||
| Lipiodol marking | Lipiodol | Pre interventional CT scan | Preoperatively, lipiodol is injected near the tumor under CT guidance. Intraoperative fluoroscopy is used to localize the tumor | AEs | Pneumothorax |
| post interventional CT scan | Radiation exposure during surgery | Intrapulmonary hemorrhage | |||
| Fluoroscopy | Air embolism | ||||
| VAL-MAP 1.0 | Dye | Pre interventional CT scan | Dye marking is usually done within 48 h of the surgery. Control by CT scan is required before surgery | Deep margin | Pneumothorax |
| Virtual planning software | Dye marking is not visible | Intrapulmonary hemorrhage | |||
| Fluoroscopy | |||||
| post interventional CT scan | |||||
| VAL-MAP 2.0 | Coil (± dye) | Pre interventional CT scan | After planning the lung map using virtual bronchoscopy, actual bronchoscopy will be conducted with sedation and local anaesthesia on the day of the operation or 1 day preoperatively | Radiation exposure during surgery | Displacement after bronchoscopic placement |
| Virtual planning software | |||||
| Fluoroscopy | |||||
| RFID lung marking | RFID tag | Pre interventional CT scan | RFID tag placement is performed 2 days before surgery using a bronchoscope reference to a fluoroscopy and cone-beam CT | Cost | Dislodgement of the RFID tag |
| Virtual planning software | |||||
| Fluoroscopy and cone-beam CT | |||||
| post interventional CT scan |
AE, adverse event; CT, computed tomography; VAL-MAP, virtual-assisted lung mapping; RFID, radiofrequency identification.
RFID lung marking systems are reliable for deep margins and easy tumor identification. The IC tag is implanted in the bronchus traveling near the tumor using a bronchoscope under general anesthesia two days before surgery. During resection, the IC tag can be detected by the surgical antenna to determine the localization of the tag. The tag detection tone changes in five steps depending on the distance from the tag. If it is possible to place the tag on the deep side of the tumor, the certainty of securing a deep margin is increased.
An institution using an RFID lung-marking system was reported in a private retrospective analysis of 39 cases. Despite the target tumor being a small pulmonary nodule with a median size of 9.0 mm (range, 8.1–12.9 mm), complete resection of all lesions was performed with definitive surgical margins. The median pathological surgical margin was 15.0 mm (range, 10–17.5 mm) (11).
Another retrospective study of 182 patients (84 males and 98 females) reported that 29 patients (15.9%) had a history of ipsilateral pulmonary resection; however, all operations were performed thoracoscopically. The mean diameter of the nodule was 10.9±5.4 mm and its depth from the lung surface was 14.6±9.9 mm. The success rate of uncomplicated resection was 100% (12). These results indicate that the RFID lung marking system is expected to be a device that will increase the surgical accuracy of surgeons by ensuring the certainty of securing deep margins, which has been an issue up to now. These results suggest that it is effective for small pulmonary nodules with adhesions in patients with a history of ipsilateral surgery, as observed in this case. In the present case, preoperative anticipation of thoracic cavity adhesions was reasonable because of the patient’s history of ipsilateral lobectomy. The specific target was pure GGN, and, if feasible, wedge resection was preferred. The tumor is characterized by its small size, deep location, and adhesion. Failure to accurately identify the tumor and establish proper margins would have necessitated complete pneumonectomy, a highly demanding procedure for the patient. To address these challenges, an RFID lung marking system was employed to accurately localize the tumor independent of adhesion-related constraints and to secure an adequate margin.
It is important to note certain limitations associated with RFID lung marking systems. The first problem with RFID lung marking systems is cost and facility equipment. Equipment must be purchased and tag placement should preferably be performed in a room equipped with fluoroscopy and cone-beam CT. Currently, only a limited number of facilities are equipped. An additional challenge arises from the requirement for adequate manpower and the necessity for patients to undergo two separate sessions of general anesthesia, contingent on the available equipment. The most important aspect of this procedure is the placement of the tag in a good position. Therefore, bronchoscopic skills are necessary, which can be difficult in cases where CT does not show bronchi in the vicinity of the tumor. Furthermore, considering that RFID technology represents a cutting-edge advancement, the assessment of its long-term prognosis is imperative in subsequent studies. In anticipation of the widespread use of this device, it remains crucial to continually accumulate cases and conduct comprehensive investigations of its long-term prognosis.
Conclusions
The results of this study indicate that the RFID lung-marking system is effective. In particular, the RFID lung marking system was shown to be a very effective device for cases involving adhesions in the thoracic cavity. Although more cases need to be accumulated, the results suggest the usefulness of the RFID lung marking system in revision surgery cases.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Seshiru Nakazawa and Hitoshi Igai) for the series “Simulation and Navigation Techniques in VATS/RATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-23-59/rc
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-23-59/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-23-59/coif). The series “Simulation and Navigation Techniques in VATS/RATS” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images/video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yi JH, Choi PJ, Bang JH, et al. Systemic air embolism after computed tomography-guided hook wire localization: two case reports and literature review. J Thorac Dis 2018;10:E59-64. [Crossref] [PubMed]
- Sato M, Kobayashi M, Kojima F, et al. Effect of virtual-assisted lung mapping in acquisition of surgical margins in sublobar lung resection. J Thorac Cardiovasc Surg 2018;156:1691-1701.e5. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Suzuki K, Watanabe SI, Wakabayashi M, et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg 2022;163:289-301.e2. [Crossref] [PubMed]
- Sarsam M, Baste JM, Thiberville L, et al. How Bronchoscopic Dye Marking Can Help Minimally Invasive Lung Surgery. J Clin Med 2022;11:3246. [Crossref] [PubMed]
- Ueda K, Uemura Y, Sato M. Protocol for the VAL-MAP 2.0 trial: a multicentre, single-arm, phase III trial to evaluate the effectiveness of virtual-assisted lung mapping by bronchoscopic dye injection and microcoil implementation in patients with small pulmonary nodules in Japan. BMJ Open 2019;9:e028018. [Crossref] [PubMed]
- Sekimura A, Funasaki A, Iwai S, et al. Thoracoscopic small pulmonary nodule detection using computed tomography-guided cutaneous marking and pleural marking. J Thorac Dis 2019;11:2745-53. [Crossref] [PubMed]
- Yang SM, Lin CK, Chen LW, et al. Combined virtual-assisted lung mapping (VAL-MAP) with CT-guided localization in thoracoscopic pulmonary segmentectomy. Asian J Surg 2019;42:488-94. [Crossref] [PubMed]
- Fumimoto S, Sato K, Hanaoka N, et al. Identification of factors affecting the surgical margin in wedge resection using preoperative lipiodol marking. J Thorac Dis 2021;13:3383-91. [Crossref] [PubMed]
- Ueda Y, Mitsumata S, Matsunaga H, et al. Use of a radiofrequency identification system for precise sublobar resection of small lung cancers. Surg Endosc 2023;37:2388-94. [Crossref] [PubMed]
- Miyahara S, Waseda R, Ueda Y, et al. Evaluation of the radiofrequency identification lung marking system: a multicenter study in Japan. Surg Endosc 2023;37:3619-26. [Crossref] [PubMed]
Cite this article as: Yoshimatsu K, Takenaka M, Hashimoto T, Tanaka K, Mori M, Kanayama M, Taira A, Kuroda K, Tanaka F. Efficacy of radiofrequency identification lung marking system in revision surgery: a case report. Video-assist Thorac Surg 2024;9:11.


