
Dual indocyanine green fluorescence imaging for segmentectomy in pulmonary metastasis of hepatocellular carcinoma: a case report
Highlight box
Key findings
• “Dual indocyanine green (ICG) fluorescence imaging”, i.e., combining tumor fluorescence by preoperative ICG administration and intersegmental plane fluorescence by intraoperative ICG administration, was useful for accurate segmentectomy for pulmonary metastasis of hepatocellular carcinoma (HCC).
What is known and what is new?
• The visibility achieved with dual ICG fluorescence imaging was confirmed for the first time in thoracoscopic surgery. Although intersegmental plane fluorescence became unclear over time, tumor fluorescence remained strong throughout the surgery.
What is the implication, and what should change now?
• By using dual ICG fluorescence for lung metastases of HCC that are close to the visceral pleura, wedge resection, and segmentectomy can be performed without marking.
Introduction
When indocyanine green (ICG) is intravenously administered more than 24 hours before surgery for hepatocellular carcinoma (HCC), it is taken up by the tumor and retained for several days (1,2). Therefore, both primary and metastatic lesions emit fluorescence when exposed to near-infrared light during surgery (3). Taking advantage of these characteristics, we reported that ICG fluorescence is useful for identifying tumors and securing sufficient margins in wedge resection of pulmonary metastases of HCC (4,5).
In recent years, ICG has frequently been used during pulmonary segmentectomy because it can be administered intraoperatively to identify the intersegmental plane (6-12).
Here, we report segmentectomy for pulmonary metastasis of HCC using “dual ICG fluorescence imaging” by combining tumor fluorescence, achieved with preoperative ICG administration, and intersegmental plane fluorescence, achieved with intraoperative ICG administration. This case is presented in accordance with the CARE reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-23-65/rc).
Case description
A 62-year-old Japanese male was referred to our department. He had a history of stage IVa HCC (pT4N0M0) and had undergone hepatectomy 5 years previously. The cancer recurred regionally immediately after the surgery and was managed using radiofrequency ablation, transarterial chemoembolization, and lenvatinib. No intrahepatic lesions remained, and lenvatinib administration had been discontinued 2 years previously. Because a solitary nodule in the right upper lobe of his lung was found a year earlier, administration of atezolizumab + bevacizumab was initiated. The number of pulmonary nodules did not decrease despite the medication. He experienced a brain stroke as an adverse event and had to cease taking the medication. As a result of vigorous rehabilitation, he was able to return to his daily activities and requested surgery for the lung tumors. Contrast-enhanced computed tomography (CT) showed a 12-mm nodule in the right ventral segment (S3), 4-mm deep from the visceral pleura (Figure 1A,1B). No metastatic lesions were observed. We diagnosed the patient with suspected metastatic lung cancer and planned to perform a right S3 segmentectomy.
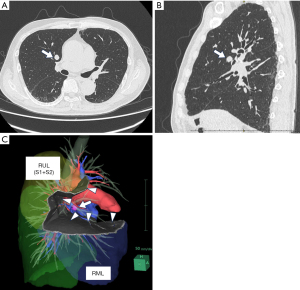
3D-CT demonstrated a surgical margin of approximately 8 mm (Figure 1C). ICG (0.5 mg/kg body weight) was injected intravenously 3 days before surgery. The surgery was conducted using three-port video-assisted thoracic surgery with an endoscopic ICG near-infrared fluorescence imaging system (OPAL1, Karl Storz, Tuttlingen, Germany). When the inside of the thoracic cavity was observed in the ICG mode, the tumor could be seen to be located under the visceral pleura. The ICG fluorescence of the tumor was visible through the pleura throughout the surgery; thus, we could perform the procedure without touching or grasping the tumor (Figure 2A). No lesions emitting ICG fluorescence were observed in the thoracic cavity other than the target tumor.
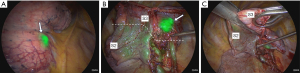
First, the right superior pulmonary vein and the pulmonary artery were exposed. A3 was cut using a linear stapler. Subsequently, V3b (running below S3b) and V3a (running between S3a and S3b) were ligated and cut, and B3 was cut using a linear stapler. V2c (running between S2b and S3a) and V1b (running between S1b and S3b) are sufficiently detached from the periphery. After cutting all blood vessels and bronchi involved in S3, ICG (0.1 mg/kg body weight) was again injected intravenously. The lung to be preserved was fluorescent, and the segment to be resected (S3) was depicted as a deficit in fluorescence. The fluorescence of the tumor was strong throughout surgery, whereas that of the intersegmental plane was moderate and diminished with time, making it easy to distinguish between the two (Figure 2B). The resection line was determined according to the fluorescence of the intersegmental plane, paying careful attention to the surgical margin because the tumor was located near the interlobar fissure. After the intersegmental plane fluorescence had attenuated, the lung parenchyma was divided using a linear stapler as planned while monitoring the tumor fluorescence (Figure 2C). The surgical time was 317 min, and blood loss was 5 cc.
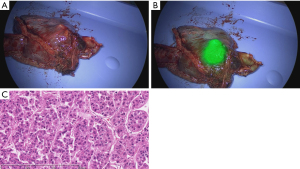
Video-assisted thoracoscopic surgery was performed on postoperative day 15 because of prolonged air leakage, and the patient was discharged on postoperative day 25. The tumor was finally diagnosed as a metastatic carcinoma (moderately differentiated HCC) by histological examination, and the surgical margin was 9 mm, including the stapler (Figure 3A-3C).
This study was approved by the Tokushima University Hospital Institutional Review Board in February 2020 (No. 3672). The opt-out method was used for this retrospective study, in which patients were included in the research unless they specifically chose to be excluded. All procedures performed in this study were conducted in accordance with the ethical standards of the institution and with the Helsinki Declaration (as revised in 2013). Publication of this case report and accompanying images was waived from patient consent in accordance with the decision of the Tokushima University Hospital Institutional Review Board in February 2020 (No. 3672).
Discussion
We had previously reported the usefulness of tumor fluorescence using preoperative ICG administration in thoracoscopic wedge resection for pulmonary metastasis of HCC (4,5). In segmentectomy, the ability to confirm localization by tumor fluorescence throughout surgery would contribute to reliable resection. In this case, we successfully performed pulmonary segmentectomy for pulmonary metastasis of HCC using “dual ICG fluorescence imaging”, which allows tumor localization based on tumor fluorescence during surgery achieved by administering ICG preoperatively, as well as identification of the intersegmental plane based on intersegmental fluorescence achieved by administering ICG intraoperatively. It was easy to distinguish between the two types of fluorescence because the tumor remained strongly fluorescent throughout the surgery. In contrast, intersegmental plane fluorescence weakened over time after intraoperative ICG administration.
Intersegmental plane identification using intraoperative ICG administration has an identification rate of approximately 90%, making it a promising method (8,9,11,12). However, its limitations include the difficulty in identifying emphysematous lungs and the slight spread of fluorescence to the resected lung over time (7). In most cases, it is associated with an increased oncological margin distance from the tumor to the staple line beyond that judged by the surgeon. However, in 10% of cases, the plane predicted by the surgeon was farther from the tumor than the actual plane identified using ICG mapping (6). Therefore, not all segmentectomy can be performed using only intersegmental plane fluorescence with intraoperative ICG administration. Even if the tumor cannot be identified on the pleural surface during surgery, if it can be identified by fluorescence imaging, it will be possible to perform a segmentectomy that can reliably include the tumor on the resected side.
In laparoscopic liver resection for HCC, the combined use of fluorescence images obtained by preoperative and intraoperative ICG administration has been reported to be useful as a real-time navigation tool (13). Here, we performed “dual ICG fluorescence imaging” in pulmonary segmentectomy, which has not been reported previously. We found that this real-time navigation tool was as useful for pulmonary segmentectomy as for liver resection.
However, the most significant limitation of the ICG fluorescence method is the depth of penetration of near-infrared light. In the case of tumors more than 1-cm deep from the pleura, observation of fluorescence is difficult; therefore, this method may only be usable in a limited number of patients (4,14).
Conclusions
In segmentectomy for pulmonary metastasis of HCC, the combination of preoperative ICG administration for visualizing tumor fluorescence and intraoperative ICG administration for visualizing intersegmental fluorescence provides excellent visibility. It contributes to reliable segmentectomy of the tumor.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Seshiru Nakazawa and Hitoshi Igai) for the series “Simulation and Navigation Techniques in VATS/RATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-23-65/rc
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-23-65/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-23-65/coif). The series “Simulation and Navigation Techniques in VATS/RATS” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Tokushima University Hospital Institutional Review Board in February 2020 (No. 3672). All procedures performed in this study were conducted in accordance with the ethical standards of the institution and with the Helsinki Declaration (as revised in 2013). Publication of this case report and accompanying images was waived from patient consent in accordance with the decision of the Tokushima University Hospital Institutional Review Board in February 2020 (No. 3672).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ishizawa T, Fukushima N, Shibahara J, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009;115:2491-504. [Crossref] [PubMed]
- Gotoh K, Yamada T, Ishikawa O, et al. A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J Surg Oncol 2009;100:75-9. [Crossref] [PubMed]
- Satou S, Ishizawa T, Masuda K, et al. Indocyanine green fluorescent imaging for detecting extrahepatic metastasis of hepatocellular carcinoma. J Gastroenterol 2013;48:1136-43. [Crossref] [PubMed]
- Kawakita N, Takizawa H, Sawada T, et al. Indocyanine green fluorescence imaging for resection of pulmonary metastasis of hepatocellular carcinoma. J Thorac Dis 2019;11:944-9. [Crossref] [PubMed]
- Kawakita N, Takizawa H, Kondo K, et al. Indocyanine Green Fluorescence Navigation Thoracoscopic Metastasectomy for Pulmonary Metastasis of Hepatocellular Carcinoma. Ann Thorac Cardiovasc Surg 2016;22:367-9. [Crossref] [PubMed]
- Mehta M, Patel YS, Yasufuku K, et al. Near-infrared mapping with indocyanine green is associated with an increase in oncological margin length in minimally invasive segmentectomy. J Thorac Cardiovasc Surg 2019;157:2029-35. [Crossref] [PubMed]
- Mun M, Okumura S, Nakao M, et al. Indocyanine green fluorescence-navigated thoracoscopic anatomical segmentectomy. J Vis Surg 2017;3:80. [Crossref] [PubMed]
- Guigard S, Triponez F, Bédat B, et al. Usefulness of near-infrared angiography for identifying the intersegmental plane and vascular supply during video-assisted thoracoscopic segmentectomy. Interact Cardiovasc Thorac Surg 2017;25:703-9. [Crossref] [PubMed]
- Tarumi S, Misaki N, Kasai Y, et al. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg 2014;46:112-5. [Crossref] [PubMed]
- Pardolesi A, Veronesi G, Solli P, et al. Use of indocyanine green to facilitate intersegmental plane identification during robotic anatomic segmentectomy. J Thorac Cardiovasc Surg 2014;148:737-8. [Crossref] [PubMed]
- Kasai Y, Tarumi S, Chang SS, et al. Clinical trial of new methods for identifying lung intersegmental borders using infrared thoracoscopy with indocyanine green: comparative analysis of 2- and 1-wavelength methods. Eur J Cardiothorac Surg 2013;44:1103-7. [Crossref] [PubMed]
- Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010;140:752-6. [Crossref] [PubMed]
- Terasawa M, Ishizawa T, Mise Y, et al. Applications of fusion-fluorescence imaging using indocyanine green in laparoscopic hepatectomy. Surg Endosc 2017;31:5111-8. [Crossref] [PubMed]
- Okusanya OT, Holt D, Heitjan D, et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg 2014;98:1223-30. [Crossref] [PubMed]
Cite this article as: Sumitomo H, Toba H, Kawakita N, Takeuchi T, Miyamoto N, Sakamoto S, Morishita A, Takizawa H. Dual indocyanine green fluorescence imaging for segmentectomy in pulmonary metastasis of hepatocellular carcinoma: a case report. Video-assist Thorac Surg 2024;9:12.


