
Uniportal VATS and hybrid VATS en bloc lung and chest wall resection—report of surgical technique and own experience
Highlight box
Surgical highlights
• Thorough staging and adequate preoperative planning (surgical approach, location of incision, extent of resection, need for reconstruction) are key before indication for radical en bloc resection in non-small cell lung cancer with chest wall infiltration is made. In case of doubt, open resection remains the standard approach for en bloc lung and chest wall resection.
What is conventional and what is novel/modified?
• Only small case series of minimally invasive en bloc lung and chest wall resection are available.
• The operative technique and decision-making processes are rarely described in detail.
• We provide a detailed step-by-step guide to support preoperative and intraoperative decision-making for minimally invasive en bloc lung and chest wall resection.
What is the implication and what should change now?
• With increasing experience in minimally invasive approaches, extended resections can be performed by minimally invasive procedures. Especially the hybrid video-assisted thoracoscopic approach is becoming more and more popular and showing good results.
Introduction
Approximately 5% of all patients presenting with diagnosis of lung cancer show chest wall invasion, either limited to the parietal pleura or invading the intercostal muscles, the ribs and vertebral bodies or extending into the soft tissue underneath (1-3).
In 1947, Coleman reported the first case series of five patients treated by en bloc resection for lung cancer invading the chest wall including long-term survival (6 years). Prior to that, chest wall infiltration was generally seen as a contraindication for upfront surgical resection (4). Ten years later, in 1957, Gronqvist et al. reported survival over 2 years (12.5%) in 2 out of 16 patients treated by either pneumonectomy or lobectomy for lung cancer involving the chest wall (5). Nevertheless, due to complication rate, difficulties in achieving complete resection in locally advanced tumors and generally lower survival rates of patients with these advanced tumors (3-5), lung cancer invading the chest wall was continuously considered as a potential contraindication for surgery for a long time (4,6,7). Later on, with the availability of diagnostics including computed tomography (CT) scan for evaluation of surrounding tissue infiltration and the routinely performed mediastinoscopy, clinical lung cancer staging was improved during the 1970s and 1980s. The use of a thoracoscope for exploration of the thoracic cavity during the 1990s and the consequential development of video-assisted thoracoscopic surgery (VATS) allowed for a minimally invasive exploration of the thoracic cavity as an additional part of local tumor staging and assessment of resectability (6). With good results being subsequently reported over the following years, thoracotomy with en bloc resection became the traditional and potential curative approach in lung cancer with chest wall involvement (6,8).
In recent years, significant technological advancements established VATS anatomic lung resection as the gold standard in treatment of stage I and II non-small cell lung cancer (NSCLC). Many studies have shown that VATS is a feasible, safe and beneficial technique for major lung resections compared to the conventional open thoracotomy approach (9-14). Moreover, newer and even less invasive methods have emerged in the past few decades. One of these methods is the single-incision VATS or uniportal VATS (uVATS), which uses only one small incision without any rib spreading to perform anatomic lung resections of almost every extent without compromising safety compared to the multiportal approach (15). Furthermore, uVATS can potentially improve perioperative outcomes, including faster recovery with shorter length of hospital stay, shortened chest tube duration and reduced overall complication rate (16-18). This, combined with novel innovations in surgical instruments, allows for minimally invasive resection of even locally advanced tumors.
Chest wall infiltration does not always mean, that parts of the boney thoracic cage must be resected. However, the depth of chest wall infiltration plays a key role in selection of the surgical approach and the extent of resection. In 2002, Burkhart et al. reported a larger case series, with 29 out of 95 (30.5%) resected lung cancer specimens showing tumor spread into the parietal pleura only, while 43 (45.3%) involved the parietal pleura and soft tissue of the chest wall and 23 (24.2%) tumors infiltrated the parietal pleura, soft and bone tissue of the chest wall (19). Hence, the extent of chest wall resection can vary between extrapleural resection up to extended resections including multi-level partial rib and even partial vertebral body resection.
Various approaches exist in minimally invasive thoracic surgery with a difference in the number of used ports and size of the respective incisions. There is also a combination of VATS with an additional counter incision (analogous to open surgery) directly above the area of chest wall infiltration (7,20-23). This hybrid VATS approach allows direct access to the tumor with an intrathoracic (VATS) and direct extrathoracic view (counter incision), facilitating chest wall resection in large tumors and ensuring safety margins, where the thoracoscopic view can be very limited. Depending on tumor size and planned extent of chest wall resection, the approach for en bloc lung and chest wall resection can vary between open resection, hybrid VATS or even complete thoracoscopic approaches, such as multiportal VATS (mVATS) and uVATS.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was not required because of the descriptive manner of this study with limited participants (no more than 5) for image use. Written informed consent was obtained from all patients for publication of this paper including all accompanying images. We present this article in accordance with the SUPER reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-23-22/rc).
Patient selection and preoperative planning
Pathological confirmation of NSCLC as well as adequate staging is essential before initiating therapy. After tumor staging is completed and nodal metastases as well as distant metastases are excluded, the need for primary tumor resection or neoadjuvant therapy should be discussed, depending on tumor stage. We recommend an interdisciplinary case discussion, such as a tumor board.
A contrast-enhanced chest CT allows an assessment of tumor size, localization, and extent of chest wall infiltration. In addition, a thoracic magnetic resonance tomography (MRT) provides valuable additional information regarding the depth of tissue infiltration and extent, allowing an accurate preoperative assessment regarding the expected extent of chest wall resection as well as selection of the surgical approach.
Pancoast tumors are handled as a separate tumor entity. They are typically not suitable for a minimally invasive approach depending on their apicoposterior location with limited exposure through the VATS approach. However, in highly selected cases, complete minimally invasive resection of Pancoast tumors including chest wall resection can be feasible, especially in robotic-assisted thoracic surgery due to better range of motion of the surgical instruments (24).
The following recommendations as shown in Figure 1 are not set in stone and should be assessed individually case by case.
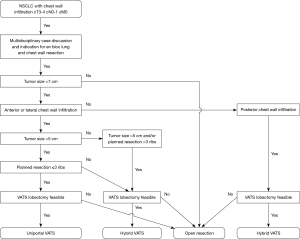
After indication for surgery is made, the thoracic surgeon has to decide about the surgical approach (open versus minimally invasive). First, this decision highly depends on surgical preference and experience in minimally invasive tumor surgery. Furthermore, tumor size and localization of suspected chest wall infiltration allow for specified planning of the surgical approach, as shown in Figure 1 and described below.
For larger tumors (>7 cm), the value of the minimally invasive technique may be questionable due to limited visualization of tumor infiltration within the thoracic cavity caused by tumor size and overall reduced overview. In such cases, primary lobectomy through VATS is challenging due to the limited mobility of the lobe caused by broad tumor adherence to the chest wall. Consequently, open resection continues to be considered the gold standard approach for en bloc lung and chest wall resections in these cases.
Smaller tumors ranging up to a size of 5 cm may also infiltrate the surrounding muscles and ribs extensively. For those located anterolaterally, VATS and especially uVATS can be a feasible approach, due to the thoracoscopic overview and easier access to the chest wall with angulated instruments as well as the possibility to use the single incision in uVATS or the utility incision in mVATS for specimen removal. Whether anatomic lung resection or chest wall resection is performed first depends on intraoperative exposure, view and mobility of the lung (see below).
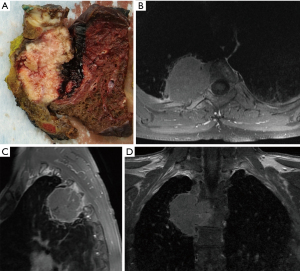
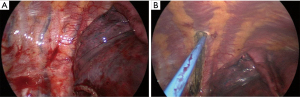
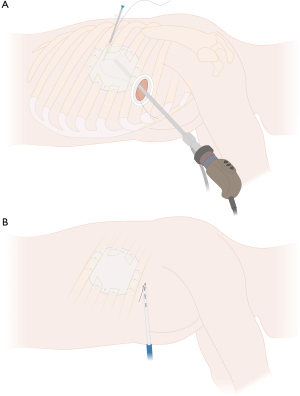
For tumors larger than 5 cm and those infiltrating the posterior chest wall or even the spine (Figure 2), hybrid VATS can be a feasible approach in selected cases. The latter can provide a very good thoracoscopic overview (Figure 3A) and facilitate chest wall resection before or after (Figure 3B) anatomic lung resection.
With the uVATS approach, up to 3–4 ribs can be partially resected. For cases where more than 3 or 4 ribs must be resected, a hybrid or even an open approach via thoracotomy can facilitate chest wall resection and may be favored.
In cases with preoperative suspicion of chest wall infiltration, chest wall or rib resection should be planned, leaving a safety margin of one rib width (approximately 2 cm) around the adhesion zone or the zone of suspected chest wall infiltration. According to the planned location and extent of resection, the location of the uVATS incision should be determined (see below).
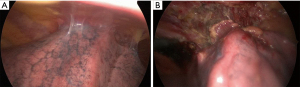
If there is no preoperative suspicion of chest wall infiltration but broad adhesions to the chest wall are found intraoperatively as shown in Figure 4, an extrapleural resection can be performed in combination with an intraoperative rapid frozen section of the main specimen or a follow-up resection to confirm complete resection or to assess the need of further resection (completion).
Intraoperative decision-making and operative steps
Based on preoperative imaging, which determines tumor size and location as well as the depth of suspected chest wall infiltration, the decision regarding the feasibility of a minimally invasive approach should be made preoperatively (see above).
In cases involving the lateral or anterior chest wall, it is possible to adapt the location of the uniportal access or the utility incision in mVATS. For example, placing the uVATS incision more anteriorly or caudally relative to the standard position (fourth or fifth intercostal space in the anterior to midaxillary line) may allow for easier access at the border of the planned chest wall resection area. A chest wall resection performed through a singular access with direct (view of the chest wall through the uniportal access) and thoracoscopic visualization is thus possible (Figure 5).
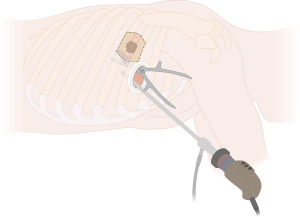
For smaller tumors up to 5 cm with an anterolateral location of chest wall involvement and good exposure/visibility of the affected chest wall by VATS, chest wall resection can be performed first. This, in turn, significantly facilitates the subsequent lobectomy by increasing the mobility of the affected lobe.
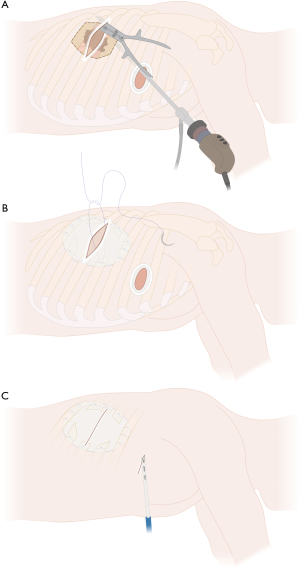
Larger tumors with a wide infiltration of the chest wall or tumors located posteriorly, which cannot be fully visualized by thoracoscopy, are better suited for a hybrid technique due to the limited thoracoscopic view of the posterior chest wall and posterior part of the lung and hilum. Hybrid VATS (Figure 6) uses an additional incision directly above the tumor and the affected chest wall area (counter incision) for chest wall resection, which can be performed either before or after VATS lobectomy (uVATS or mVATS). Large or posteriorly located tumors that may be challenging in their resection can be initially inspected by thoracoscopy (uVATS or mVATS), allowing an accurate assessment of the extent of chest wall resection. However, it can be challenging to perform a lobectomy due to the limited mobility of the lung. Hence, chest wall resection can be performed prior to lobectomy or in difficult cases, conversion to open resection is reasonable to allow complete and en bloc resection.
Different VATS approaches
Depending on the surgeon’s preference, anatomic lung resections can be performed either by uVATS or mVATS. Both techniques use a 3–4 cm incision, which can be easily enlarged for removal of larger specimens (lung and adherent chest wall), if needed. The advantages of uVATS have been shown in several studies (15-17,25). The risk of postoperative intercostal neuralgia is increased with additional ports (26) and, in general, uVATS is associated with lower postoperative pain compared to mVATS, due to the involvement of only one intercostal space. However, both, uVATS and mVATS can be used for lung and chest wall resections and mVATS can also be performed using only one intercostal space (e.g., in biportal VATS).
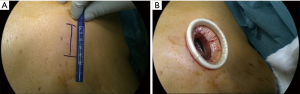
We use the standard technique for the uVATS approach in anatomic lung resection with the patient receiving general anesthesia with a double-lumen endotracheal tube (DLT) or an endobronchial blocker. The patient is then placed in a lateral decubitus position. The surgeon as well as the first assistant are positioned on the ventral side of the patient. A single, muscle-sparing incision of 3–4 cm in the anterior to midaxillary line of the fourth or fifth intercostal space is used to access the thoracic cavity (Figure 7A). Depending on the targeted lobe or segments as well as the area of the chest wall resection, the location of the incision can vary (see above). The routine use of a soft wound protector maximizes wound exposure without the need for rib spreading (Figure 7B). It simultaneously protects the wound from contamination and avoids soiling the camera. We usually use a 5 mm 30° angled thoracoscope for uVATS procedures. Vascular and bronchial dissection can be performed analogously to any other thoracoscopic anatomic resection approach (18).
For large tumors, posterior location, large chest wall resections or extended resections including partial vertebral body resection, an additional incision directly above the area of the involved chest wall (verified through thoracoscopy) can facilitate en bloc chest wall resection as shown in Figure 6. At the same time, this hybrid approach allows for effortless large specimen removal en bloc with the attached lung tissue through the newly created chest wall defect and the superjacent utility or counter incision. Compared to the open approach with thoracotomy, this method does not require any rib spreading or scapular mobilization. Thus, the benefits of minimally invasive surgery still apply.
For the hybrid approach, depending on the location of chest wall infiltration and the respective counter incision, the patient can stay in the lateral decubitus position in most cases. Chest wall resection can then be performed analogously to open resection. For tumors invading the chest wall dorsally, such as shown in Figure 2, the patient can also be repositioned after anatomic lung resection was performed by uVATS.
Figure 3 shows a case with a resected right upper lobe attached to the chest wall, prior to chest wall and partial vertebral body resection. Subsequently, prone position, as traditionally used in laminectomy or spondylodesis, allows for a direct posterior approach to the thoracic spine and completion of the en bloc resection in such cases.
Resection
The location of the tumor and the involved chest wall structures determine the further approach of the minimally invasive chest wall resection as illustrated in Figures 5,6,8-10 and described above.
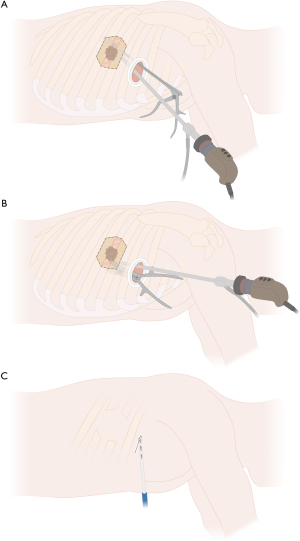
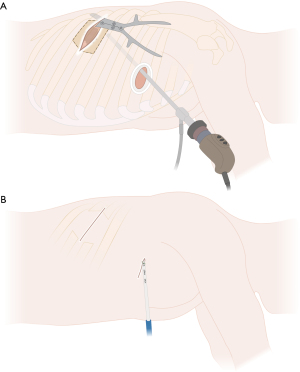
In case the lung cannot be separated from the chest wall through extrapleural mobilization and/or chest wall infiltration is present beyond the parietal pleura, the procedure can be continued either by completing anatomic lung resection prior to chest wall resection or vice versa. Performing the chest wall resection first might optimize the thoracoscopic view and can facilitate hilar or interlobar dissection due to more mobility of the lobe with the attached part of the resected chest wall, especially in case of chest wall involvement close to the single-port or if the attached lung to the chest wall blocks direct access to the hilum or the fissure. However, performing lung resection prior to chest wall resection can provide better exposure of the posterior aspect of the ribs due to greater mobilization of the dissected lung, which remains attached to the chest wall as shown in Figure 3A. In case of difficult exposure through uVATS, conversion to hybrid VATS or even thoracotomy can be performed.
After the required extent of chest wall resection is determined to obtain sufficient safety margins, an angled cautery or an energy device can be used to mark and finally divide the intercostal muscles through the single-port access (Figure 3B). We generally use an energy device (e.g., LigaSure™) to incise the parietal pleura and to divide the intercostal muscles as well as the vascular and neural structures after carefully detaching them from the overlaying rib. Figure 8 illustrates the division of the chest wall area to be resected without rib spreading and without an additional incision.
Next, in case of involvement of the boney structures of the chest wall, ribs have to be resected. Endoscopic instruments or bone cutters for open resection can be used as shown in Figure 5 and Figure 8A,8B.
Depending on the location of chest wall involvement, endoscopic instruments including bone cutters may facilitate navigating difficult angles. The division of the anterior and posterior rib is performed by using a rongeur, Liston bone cutter or guillotine rib shear, placed through the single-port, without additional incision (Figure 8A,8B). In case the uniportal access lies within or adjacent to the area of chest wall resection, chest wall resection can still be performed through the same access as shown in Figure 5. The energy device is then used to remove any attachments to the overlying serratus and latissimus muscles. This leaves a hole in the chest wall with completely intact serratus anterior and latissimus dorsi.
If lung resection follows, the lung with the adjacent chest wall can be placed in the thoracic cavity to continue lung resection with a mobile lobe. In case lung resection has already been performed, the specimen (lung en bloc with attached chest wall) can then be removed from the pleural cavity through the single-port access with the ribs getting out first. Depending on specimen size, in some cases, a small enlargement of the skin incision is needed for specimen retrieval. Due to the cutting edges of the resected ribs, we routinely use the tear resistant and impermeable Eco Sac Tissue Retrieval Bag [Fannin (UK) Ltd., Measham UK], which is much more robust compared to the normal self-opening, detachable and transparent retrieval bags. The specimen is then sent for final histopathological evaluation or, if needed, for frozen section examination of resection margins. The oncological resection is then completed by radical and complete mediastinal lymph node dissection (MLND). If necessary, chest wall reconstruction is performed next as shown in Figure 6B for hybrid VATS and in Figure 9A for complete uVATS, followed by chest tube placement and wound closure layer by layer (Figure 6C).
Soft tissue coverage and chest wall reconstruction methods
Depending on the size of the created chest wall defect, the surrounding soft tissue can cover the defect sufficiently to prevent lung hernia and the adjacent ribs may provide enough stabilization without the need for chest wall reconstruction (Figure 8C and Figure 10B). Therefore, for small chest wall defects, the necessity of reconstruction depends on case by case and is not always mandatory. Especially posterior defects and defects up to two ribs or 5 cm in diameter can be left without reconstruction. Both options (reconstruction and non-reconstruction) are shown in Figure 9B and Figure 8C for complete uVATS and in Figure 6C and Figure 10B for hybrid VATS.
For larger chest wall defects (three or more ribs partially resected or defect >5 cm in diameter), chest wall reconstruction should be performed using a mesh, e.g., a non-absorbable synthetic woven mesh (polypropylene mesh), as shown in Figure 6B and Figure 9A. Chest wall reconstruction can be performed in complete uVATS technique by thoracoscopic suturing or using an endoscopic suturing device, such as the Endo Close™, as shown in Figure 9A. We do not routinely use tack fixation to hold the mesh in place, as tacks placed into the intercostal space or close to the neurovascular bundle can cause postoperative bleeding or nerve lesions, leading to chronic postoperative pain. If not placed correctly, these tacks can also migrate, with a potential need for thoracoscopic removal. For hybrid VATS chest wall reconstruction, the mesh can be attached by non-absorbable sutures through the created counter incision analogous to reconstruction in open surgery as shown in Figure 6B.
Postoperative considerations
Compared to conventional lobectomy without chest wall resection, patients with additional chest wall resection show increased morbidity (20%) and mortality (4–15%) (6,27). Both minimally invasive and open chest wall resection result in local postoperative pain mostly due to chest wall resection itself and not due to thoracotomy and rib spreading. Postoperative pain can be well controlled by epidural analgesia through a thoracic epidural catheter in the first few days. However, many years of experience with VATS show advantages of the minimally invasive surgical technique beyond lesser pain.
Discussion
The beginning and evolution of chest wall resection
In recent decades, the standard approach for chest wall resection was thoracotomy (22). This approach allowed an exploratory thoracotomy, through which the extent of chest wall invasion and planned resection could be determined visually and by digital palpation (22). Since 1991, thoracoscopic exploration has been increasingly used in lung cancer surgery as the first step in assessing resectability, resulting in a decrease in overall numbers of exploratory thoracotomies performed (6).
A study by Roviaro et al. shows the technical progress in open surgery for NSCLC with chest wall infiltration and also an improved survival rate between 1970 and 1999 (6). Between 1970 and 1979, 10 out of 32 patients underwent exploratory thoracotomy and 22 underwent radical tumor resection with a mean 5-year survival rate of 22.7%. With advances in anesthesia and diagnostics, such as computed tomography in the 1970s as well as technical advances in surgery, only two exploratory thoracotomies and two exploratory thoracoscopies were performed in 47 patients between 1990 and 1999. Radical resection was performed in 43 patients with a mean 5-year survival rate of 42.7% during the same decade (6). For NSCLC with infiltration of the chest wall and without lymph node involvement, a 5-year survival rate of 40–50% after complete resection still applies today (20,28-31).
These extended thoracic resections including chest wall resection through thoracotomy are associated with significantly increased morbidity (20%) and mortality (4–15%) compared to conventional anatomic lung resection via thoracotomy (6,27).
The era of VATS
Since the beginning of the 1990s, thoracoscopy has developed not only in the context of diagnostics, but also increasingly as a therapeutic approach for anatomic lung resection. The minimally invasive technique of lung surgery in early-stage NSCLC has gained acceptance over the last two decades due to development in surgical technique and instrumentation and therefore, replaced the conventional thoracotomy as the standard approach for anatomic lung resection in early-stage NSCLC (12,32,33).
uVATS anatomic lung resections are considered technically demanding procedures. Nevertheless, a distinct learning curve can be observed, especially in centers, where uVATS is standardly used for segmentectomy, lobectomy and even for pneumonectomy or bronchial and vascular sleeve resections as in our institution. There is also an increasing trend towards VATS used for anatomic lung resection in locally advanced NSCLC (e.g., stage IIIA), provided that principles of surgical oncology can be followed and complete (R0) en bloc resection is possible (12,23,32-35).
Experience with VATS anatomic lung resections over many years has shown advantages as described in several studies, which can be explained mainly by significantly less postoperative pain and better pulmonary function. After chest wall resection, postoperative pain is mainly caused by chest wall resection itself and not by the surgical approach. Patients with chest wall resection show similar postoperative pain, whether the resection was performed by thoracotomy with rib spreading or by VATS, therefore relativizing the benefit of VATS regarding postoperative pain. Other benefits of VATS are earlier chest tube removal and shorter hospital length of stay, less inflammatory reaction with less surgical trauma overall and thus fewer postoperative complications such as atrial fibrillation and postoperative pneumonia (20,30,36-38).
Different approaches in extended VATS resection
The first description of VATS lung resection en bloc with chest wall resection was published by Widmann et al. in 2000 (27). The resection was performed through a three-port access for NSCLC with previous neoadjuvant radiation (27). Different approaches and techniques are described in single case reports and smaller case series in the literature (20-22,27,39,40). These experienced thoracic surgeons have reported their surgical technique with different numbers and placement of ports, ranging from uniportal (40) up to five-port access (41) as well as using a combined hybrid approach (7,20-23), as described above.
Different techniques exist for VATS regarding number of ports and their location or placement. Compared to the traditional multiport approach, uVATS anatomic resections can be performed without compromising surgical safety through a single small incision, using only one intercostal space without rib spreading (15,18,25). Therefore, the single-port approach with its angled instruments offers great potential for extended anatomic resection as well as easier specimen removal (even when a part of the chest wall is attached to the lung) through the standard 3–4 cm or slightly enlarged incision.
Berry et al. described a hybrid approach in which the thoracoscopic technique is combined with a counter incision to perform the en bloc chest wall resection (20). Gonzalez-Rivas et al. also described a hybrid technique, illustrated in a case, where a right upper lobe resection was performed by uVATS with an additional posterior incision to resect the fourth and fifth rib posteriorly (21).
Rib resection and chest wall reconstruction
Most thoracoscopic rib resections described in the literature are located posteriorly. Limited posterior partial rib resection does not require thoracic wall reconstruction, as the overlying scapula and dense dorsal muscles seal a small defect (8,21,28,39).
Anteriorly to laterally, there is a thinner muscle coverage of the rib cage with higher risk of postoperative flail chest with respiratory insufficiency or pulmonary hernia (8). In anterior and lateral chest wall resections, there are no clear guidelines for reconstruction. According to the literature, reconstruction of the chest wall is recommended if two or more ribs are partially resected or if the defect exceeds 5 cm in diameter (39,42-44).
There exists a wider choice of non-absorbable prosthetic material for chest wall reconstruction, such as polytetrafluoroethylene (PTFE) meshes (e.g., Teflon), expanded PTFE meshes (e.g., Gore-Tex) and polypropylene meshes (e.g., Prolene) (6,45). The PTFE mesh and the polypropylene mesh are the most commonly used meshes (39,45).
As described above, we do not routinely recommend the use of tack fixation due to the risk of intercostal bleeding and nerve lesions. Furthermore, tack migration can occur with risk of erosion and bleeding, resulting in a need for surgical removal of the migrated foreign body. Therefore, we recommend using an endoscopic fixation tool, for example, the Endo Close™ endoscopic suturing device as illustrated in Figure 9A.
Limits of VATS in chest wall resection
The minimally invasive technique using VATS for chest wall resection has shown its limits, especially in very large tumors over 7 cm and in superior sulcus tumors. Superior sulcus or Pancoast tumors are generally seen as a separate entity of lung tumors with different approaches in treatment. It is difficult to perform VATS resection in cases with tumors >7 cm because the large tumor mass limits the mobility of the lung and can obstruct the view of both the area of chest wall infiltration and the central hilar structures (30). In large tumors, surgery with inadequate visibility is not only very demanding but also carries a high intraoperative risk of inadvertent vascular injury. In addition, removal of the tumor en bloc leads to a certain enlargement of the incision with the need for rib spreading, losing some of the benefits of the minimally invasive approach. Reconstruction of very large thoracic wall defects is also shown to be unsuitable for VATS techniques.
Postoperative complications
Some of the postoperative complications reported in some case series of minimally invasive chest wall resection in the literature most frequently include cardiac arrhythmias such as atrial fibrillation, prolonged air leak and postoperative pneumonia (20,23,46,47). Overall, the types of postoperative complications are similar to minimally invasive lung resection without chest wall resection, but according to the greater extent of surgery due to chest wall resection, the risk for postoperative complications is slightly higher.
The impact of neoadjuvant treatment in minimally invasive thoracic surgery
For potentially resectable tumors (T3N1 or even T4N0–1, depending on extent) and provided that complete resection (R0) can be performed, a combined modality approach including radical en bloc resection should be discussed in selected cases after multidisciplinary evaluation. Chemotherapy can be offered in a neoadjuvant or adjuvant regime with or without radiation (34,48-53). Neoadjuvant treatment may generally accomplish a reduction in tumor size, making radical resection more feasible and can eradicate micrometastatic disease. Neoadjuvant treatment also significantly improves overall survival, time to distant recurrence and recurrence-free survival (53-62). Moreover, for superior sulcus tumors, as a separate entity of lung tumors with a different approach in therapy, anatomic lung resection following neoadjuvant chemoradiotherapy in selected patients shows the best survival benefit (50,53,63,64). However, neoadjuvant treatment induces local tissue inflammation, resulting in tissue adhesions with increased tissue and especially vascular fragility, therefore, making surgery, especially minimally invasive approaches, more difficult and adhesiolysis as well as dissection more tedious, resulting in longer operation times for open and for minimally invasive thoracic surgery (53,65-68).
Restaging, reevaluation and especially surgical planning after neoadjuvant treatment must be emphasized, as reported complete (R0) resection rates are 64% for T3N0 and 39% for T4N0 tumors (69). For an individual subgroup of patients with T3 or even T4 tumors, invading the chest wall, minimally invasive resection can be offered if deemed possible without compromising oncological radicality (53). Additionally, as experience with video-assisted and robot-assisted thoracic surgery grew over the years, neoadjuvant treatment is no longer a contraindication for minimally invasive surgery.
Own experience and expertise
Over five years (January 2016 to December 2020), 2.0% of all anatomic lung resections at our institution included en bloc chest wall resection that went beyond extrapleural mobilization of the lung. In 17.7% of these cases, indication for minimally invasive resection was made. Similar results regarding the ratio between open and minimally invasive approaches for chest wall resections are shown in the case series of Berry et al. (20), where 93 out of 105 patients were treated by thoracotomy and in 12 cases a hybrid thoracoscopic approach was used. This corresponds to 11.4% of all chest wall resections performed minimally invasive. Thus, it must be emphasized that only selected cases are suitable for such a minimally invasive approach. In addition, experience in oncological thoracic surgery, especially with minimally invasive techniques and the surgeon’s preference for these demanding resections also play a role in the decision process of indication for minimally invasive resection. Furthermore, with greater experience in minimally invasive approaches, extended resections, especially through hybrid VATS, become more popular.
In our institution, the main reason for the open approach was tumor size and preoperative neoadjuvant chemoradiotherapy or neoadjuvant chemotherapy.
In many case series reported in the literature, the surgical technique has not been discussed in detail (47) and there are also no clear descriptions of whether the chest wall resection was only an extrapleural mobilization of the lung without chest wall resection that goes beyond, therefore, making interpretation of this mixed data and the described outcomes of this heterogenous group very difficult (20,47). Especially, as depth of chest wall infiltration is a main predictive factor for prognosis and long-term survival (4,70).
Conclusions
Ongoing improvements in techniques and instruments for VATS have allowed lung surgery to become more minimally invasive with faster postoperative rehabilitation. Thus, offering minimally invasive resection for an individual subgroup of patients with lung cancer and chest wall involvement. The described approaches (uVATS and hybrid VATS) are feasible and some of the least invasive approaches in thoracic surgery for extended tumor resection.
As experience in minimally invasive thoracic surgery increases, the tendency of used approaches in extended resections shifts from open to hybrid to a more and more minimally invasive approach, with low morbidity and complication rates.
In highly selected cases with adequate preoperative planning, uniportal and hybrid VATS en bloc lung and chest wall resections are feasible therapeutic options in patients with lung cancer and chest wall involvement.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Carlos Galvez Munoz and Paula A. Ugalde Figueroa) for the series “Advanced Uniportal VATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-23-22/rc
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-23-22/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-23-22/coif). The series “Advanced Uniportal VATS” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was not required because of the descriptive manner of this study with limited participants (no more than 5) for image use. Written informed consent was obtained from all patients for publication of this paper including all accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McCaughan BC, Martini N, Bains MS, et al. Chest wall invasion in carcinoma of the lung. Therapeutic and prognostic implications. J Thorac Cardiovasc Surg 1985;89:836-41. [Crossref] [PubMed]
- Anderson BO, Burt ME. Chest wall neoplasms and their management. Ann Thorac Surg 1994;58:1774-81. [Crossref] [PubMed]
- Downey RJ, Martini N, Rusch VW, et al. Extent of chest wall invasion and survival in patients with lung cancer. Ann Thorac Surg 1999;68:188-93. [Crossref] [PubMed]
- Coleman FP. Primary Carcinoma of the Lung, with Invasion of the Ribs: Pneumonectomy and Simultaneous Block Resection of the Chest Wall. Ann Surg 1947;126:156-68. [Crossref] [PubMed]
- Gronqvist YK, Clagett OT, Mcdonald JR. Involvement of the thoracic wall in bronchogenic carcinoma; study of 16 cases in which pneumonectomy or lobectomy and simultaneous resection of the thoracic wall were done. J Thorac Surg 1957;33:487-95. [Crossref] [PubMed]
- Roviaro G, Varoli F, Grignani F, et al. Non-small cell lung cancer with chest wall invasion: evolution of surgical treatment and prognosis in the last 3 decades. Chest 2003;123:1341-7. [Crossref] [PubMed]
- Dal Agnol G, Oliveira R, Ugalde PA. Video-assisted thoracoscopic surgery lobectomy with chest wall resection. J Thorac Dis 2018;10:S2656-63. [Crossref] [PubMed]
- Martini N, McCormack P, McCaughan BC. Tumors of the Chest Wall. In: Wu Y, Peters RM. editors. International Practice in Cardiothoracic Surgery. Dordrecht: Springer Netherlands; 1986:279-90.
- Cheng D, Downey RJ, Kernstine K, et al. Video-assisted thoracic surgery in lung cancer resection: a meta-analysis and systematic review of controlled trials. Innovations (Phila) 2007;2:261-92. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- West D, Rashid S, Dunning J. Does video-assisted thoracoscopic lobectomy produce equal cancer clearance compared to open lobectomy for non-small cell carcinoma of the lung? Interact Cardiovasc Thorac Surg 2007;6:110-6. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Sihoe AD. Reasons not to perform uniportal VATS lobectomy. J Thorac Dis 2016;8:S333-43. [PubMed]
- Flury DV, Kocher GJ, Lutz JA, et al. Uniportal thoracoscopic surgery for pulmonary arteriovenous malformations—report of technique and case series. Curr Chall Thorac Surg 2020;2:26. [Crossref]
- Burkhart HM, Allen MS, Nichols FC 3rd, et al. Results of en bloc resection for bronchogenic carcinoma with chest wall invasion. J Thorac Cardiovasc Surg 2002;123:670-5. [Crossref] [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. Feasibility of hybrid thoracoscopic lobectomy and en-bloc chest wall resection. Eur J Cardiothorac Surg 2012;41:888-92. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Single-incision thoracoscopic right upper lobectomy with chest wall resection by posterior approach. Innovations (Phila) 2013;8:70-2. [Crossref] [PubMed]
- Bayarri CI, de Guevara AC, Martin-Ucar AE. Initial single-port thoracoscopy to reduce surgical trauma during open en bloc chest wall and pulmonary resection for locally invasive cancer. Interact Cardiovasc Thorac Surg 2013;17:32-5. [Crossref] [PubMed]
- Jaus MO, Forcione A, Gonfiotti A, et al. Hybrid treatment of T3 chest wall lung cancer lobectomy. J Vis Surg 2018;4:32. [Crossref] [PubMed]
- Kocher GJ, Deckarm SA, Flury DV. Completely portal robotic Pancoast tumor resection with en bloc resection of left upper lobe and chest wall. Multimed Man Cardiothorac Surg 2023; in press. [PubMed]
- Alan DLS. The Evolution of VATS Lobectomy. In: Paulo FGC. editor. Topics in Thoracic Surgery. Rijeka: IntechOpen; 2012.
- Matsuura N, Igai H, Ohsawa F, et al. Uniport vs. multiport video-assisted thoracoscopic surgery for anatomical lung resection-which is less invasive? J Thorac Dis 2021;13:244-51. [Crossref] [PubMed]
- Widmann MD, Caccavale RJ, Bocage JP, et al. Video-assisted thoracic surgery resection of chest wall en bloc for lung carcinoma. Ann Thorac Surg 2000;70:2138-40. [Crossref] [PubMed]
- Caruana EJ, Solli P, Coonar AS. Hybrid video-assisted thoracoscopic surgery lobectomy and en-bloc chest wall resection for non-small cell lung cancer. J Thorac Dis 2016;8:E935-7. [Crossref] [PubMed]
- Giaccone A, Solli P, Pardolesi A, et al. Video-assisted thoracoscopic surgery en bloc chest wall resection. J Vis Surg 2017;3:73. [Crossref] [PubMed]
- Kara HV, Balderson SS, D'Amico TA. Challenging cases: thoracoscopic lobectomy with chest wall resection and sleeve lobectomy-Duke experience. J Thorac Dis 2014;6:S637-40. [PubMed]
- Rocco G. Chest wall resection and reconstruction according to the principles of biomimesis. Semin Thorac Cardiovasc Surg 2011;23:307-13. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23. [PubMed]
- Shaw JP, Dembitzer FR, Wisnivesky JP, et al. Video-assisted thoracoscopic lobectomy: state of the art and future directions. Ann Thorac Surg 2008;85:S705-9. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:497-530. [Crossref] [PubMed]
- Hanna JM, Berry MF, D'Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis 2013;5:S182-9. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Reichert M, Kerber S, Amati AL, et al. Total video-assisted thoracoscopic (VATS) resection of a left-sided sulcus superior tumor after induction radiochemotherapy: video and review. Surg Endosc 2015;29:2407-9. [Crossref] [PubMed]
- Demmy TL, Nwogu C. Is video-assisted thoracic surgery lobectomy better? Quality of life considerations. Ann Thorac Surg 2008;85:S719-28. [Crossref] [PubMed]
- Abicht TO, de Hoyos AL. Chest wall resection and reconstruction: a true thoracoscopic approach. Innovations (Phila) 2011;6:399-402. [Crossref] [PubMed]
- Gonzalez-Rivas D, Xie B, Yang Y, et al. Uniportal video-assisted thoracoscopic lobectomy with en bloc chest wall resection. J Vis Surg 2015;1:7. [PubMed]
- Yamamoto S, Sogabe M, Endo S. Video-assisted thoracoscopic surgery lobectomy and en bloc resection of the chest wall with incision of the costovertebral joints for non-small cell lung cancer. J Surg Case Rep 2021;2021:rjab190. [Crossref] [PubMed]
- Mansour KA, Thourani VH, Losken A, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg 2002;73:1720-5; discussion 1725-6. [Crossref] [PubMed]
- Deschamps C, Tirnaksiz BM, Darbandi R, et al. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg 1999;117:588-91; discussion 591-2. [Crossref] [PubMed]
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85. [Crossref] [PubMed]
- Arnold PG, Pairolero PC. Chest-wall reconstruction: an account of 500 consecutive patients. Plast Reconstr Surg 1996;98:804-10. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Minimally invasive chest wall resection: sparing the overlying, uninvolved extrathoracic musculature of the chest. Ann Thorac Surg 2012;94:1744-7. [Crossref] [PubMed]
- Hennon MW, Dexter EU, Huang M, et al. Does Thoracoscopic Surgery Decrease the Morbidity of Combined Lung and Chest Wall Resection? Ann Thorac Surg 2015;99:1929-34; discussion 1934-5. [Crossref] [PubMed]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Prävention, Diagnostik, Therapie und Nachsorge des Lungenkarzinoms, Langversion 2.1. AWMF-Registernummer: 020/007OL. [published December 2022, accessed 18 February 2023]. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Lungenkarzinom/Version_2/LL_Lungenkarzinom_Langversion_2.1.pdf
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-99S.
- Rusch VW. Management of Pancoast tumours. Lancet Oncol 2006;7:997-1005. [Crossref] [PubMed]
- Narayan S, Thomas CR Jr. Multimodality therapy for Pancoast tumor. Nat Clin Pract Oncol 2006;3:484-91. [Crossref] [PubMed]
- Flury DV, Minervini F, Kocher GJ. Heterogeneity of stage IIIA non-small cell lung cancer—different tumours, different nodal status, different treatment, different prognosis: a narrative review. Curr Chall Thorac Surg 2022;4:13. [Crossref]
- Martins RG, D'Amico TA, Loo BW Jr, et al. The management of patients with stage IIIA non-small cell lung cancer with N2 mediastinal node involvement. J Natl Compr Canc Netw 2012;10:599-613. [Crossref] [PubMed]
- Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [Crossref] [PubMed]
- Pisters KM, Vallières E, Crowley JJ, et al. Surgery with or without preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol 2010;28:1843-9. [Crossref] [PubMed]
- Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. [Crossref] [PubMed]
- Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 2007;369:1929-37. [Crossref] [PubMed]
- Burdett SS, Stewart LA, Rydzewska L. Chemotherapy and surgery versus surgery alone in non-small cell lung cancer. Cochrane Database Syst Rev 2007;CD006157. [Crossref] [PubMed]
- Rosell R, Gómez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med 1994;330:153-8. [Crossref] [PubMed]
- Pisters K, Vallieres E, Bunn P, et al. S9900: A phase III trial of surgery alone or surgery plus preoperative (preop) paclitaxel/carboplatin (PC) chemotherapy in early stage non-small cell lung cancer (NSCLC): Preliminary results. J Clin Oncol 2005;23:LBA7012. [Crossref]
- Rusch VW, Albain KS, Crowley JJ, et al. Surgical resection of stage IIIA and stage IIIB non-small-cell lung cancer after concurrent induction chemoradiotherapy. A Southwest Oncology Group trial. J Thorac Cardiovasc Surg 1993;105:97-104; discussion 104-6. [Crossref] [PubMed]
- Wen J, Liu D, Chen D, et al. Treatment of clinical T4 stage superior sulcus non-small cell lung cancer: a propensity-matched analysis of the surveillance, epidemiology, and end results database. Biosci Rep 2019;39:BSR20181545. [Crossref] [PubMed]
- Xue Z, Wu F, Pierson KE, et al. Survival in Surgical and Nonsurgical Patients With Superior Sulcus Tumors. Ann Thorac Surg 2017;104:988-97. [Crossref] [PubMed]
- Van Schil PE, Yogeswaran K, Hendriks JM, et al. Advances in the use of surgery and multimodality treatment for N2 non-small cell lung cancer. Expert Rev Anticancer Ther 2017;17:555-61. [Crossref] [PubMed]
- Yang Z, Zhai C. Uniportal video-assisted thoracoscopic surgery following neoadjuvant chemotherapy for locally-advanced lung cancer. J Cardiothorac Surg 2018;13:33. [Crossref] [PubMed]
- Huang J, Xu X, Chen H, et al. Feasibility of complete video-assisted thoracoscopic surgery following neoadjuvant therapy for locally advanced non-small cell lung cancer. J Thorac Dis 2013;5:S267-73. [PubMed]
- Park JS, Kim HK, Choi YS, et al. Unplanned conversion to thoracotomy during video-assisted thoracic surgery lobectomy does not compromise the surgical outcome. World J Surg 2011;35:590-5. [Crossref] [PubMed]
- Rusch VW, Parekh KR, Leon L, et al. Factors determining outcome after surgical resection of T3 and T4 lung cancers of the superior sulcus. J Thorac Cardiovasc Surg 2000;119:1147-53. [Crossref] [PubMed]
- Voltolini L, Rapicetta C, Luzzi L, et al. Lung cancer with chest wall involvement: predictive factors of long-term survival after surgical resection. Lung Cancer 2006;52:359-64. [Crossref] [PubMed]
Cite this article as: Flury DV, Diezi M, Lutz JA, Kocher GJ. Uniportal VATS and hybrid VATS en bloc lung and chest wall resection—report of surgical technique and own experience. Video-assist Thorac Surg 2023;8:45.


