Scoping review on long term oncologic outcomes in robotic-assisted lobectomy
Highlight box
Key findings
• RATS lobectomy is safe and effective for oncologic resection, and DFS and lymph node harvest appears superior as compared to VATS.
What is known and what is new?
• RATS lobectomy has been gaining popularity and has shown excellent short-term perioperative outcomes.
• This manuscript aims to demonstrate the long-term oncologic outcomes from RATS lobectomy compared to published outcomes from VATS lobectomy.
What is the implication, and what should change now?
• More studies are needed to study overall survival and recurrence rates from RATS lobectomy.
Introduction
Background
Robotic-assisted surgery has changed the landscape of thoracic surgery over the last 2 decades. Open surgery was once the gold standard treatment for lobectomy, but in the 1990s, video-assisted thorascopic surgery (VATS) quickly became a popular as the minimally invasive surgical approach in pulmonary resections (1-3). However, adoption in many regions plateaued due to various factors, and starting in the early 2000s, robotic-assisted thorascopic surgery (RATS) began gaining ground. Early evidence focused initially on the safety and feasibility of this novel technique (1-4). Melfi et al. published a small case series in 2002 assessing the clinical feasibility of RATS in various lung resections using the Intuitive da Vinci system and found that while operative visualization was optimal and no technical errors occurred, operative times were much longer as compared to open or VATS approaches, and 3 of the 12 patients required conversion to a small thoracotomy (1). Similarly in 2006, Park et al. published results from a prospective study of 34 patients undergoing robotic lobectomy and concluded that operative outcomes were comparable to VATS lobectomy, though with longer operative times and higher conversion rates (2). These early studies focused on short-term outcomes such as operative time, conversions, and postoperative recovery. Over the last 15 years, rapid advancements within robotic surgery itself, including the continued evolution of the robotic surgical system, has only continued to flatten surgeon learning curves and ameliorate prior technical issues.
Rationale and knowledge gap
With the establishment of safety and feasibility of RATS from these early studies, RATS gained traction in thoracic oncology. Accordingly, the clinical question has evolved from the initial focus on safety and feasibility to a focus on long term cancer-specific outcomes and overall survival (OS), particularly as the collective experience has grown and long-term patient follow up can now be analyzed.
Objective
This review article aims to summarize contemporary data on the current state of robotic lobectomy as a maturing procedure, with a focus on oncologic outcomes and long-term survival as compared to traditional VATS techniques. We present this article in accordance with the PRISMA-ScR reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-23-26/rc).
Methods
We performed a systematic search in PubMed for Level I and II evidence, as defined by the Center of Evidence-Based Medicine (5), using the following MeSH terms and keywords: lobectomy, video-assisted thorascopic surgery, robotic-assisted thorascopic surgery, survival, recurrence. Accepted publications in the English language from January 2000 to February 2023 were included. Inclusion criteria were all comparative analyses, meta-analyses, systematic reviews, and randomized controlled trials (RCT) that reported any of the following variables: OS, disease-free survival (DFS), recurrence, lymph node counts, lymph node stations, and upstaging. Exclusion criteria were single center studies, Level III or higher studies, and studies missing all of the aforementioned variables. There was a medical librarian who assisted with gathering relevant sources, and two independent reviewers who screened each record and report retrieved for the aforementioned inclusion criteria.
Our primary outcome of interest was OS, DFS, and recurrence rates for surgically-treated lung cancer. We also analyzed surrogate markers to assess oncologic quality and used variables such as lymph node harvest and upstaging rate as quality indicators. Hazard ratios (HRs), standardized mean difference (SMD), and weighted mean difference (WMD) with 95% confidence intervals (CI) were used to represent survival data, whereas odds ratios (ORs) were used to describe dichotomous variables. Statistical heterogeneity was quantified using the I2 value. P values <0.05 were considered significant.
Results
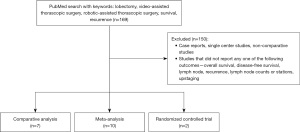
Prior to 2020, there were few large studies comparing long term outcomes from robotic lobectomy versus VATS, and the best published literature consisted of retrospective studies from several high volume centers (3,4). In the past few years, however, the amount of data focused on survival has increased significantly (6,7). Our literature search generated 169 studies, of which 19 met inclusion criteria. Most studies were excluded due to level of evidence being below our threshold. The studies included were comprised of 7 comparative analyses, 10 meta-analyses, and 2 RCTs (Figure 1). Our results will review findings from the 19 studies, categorized by the variable reported (Table 1).
Table 1
| Study | Year published | Database | Sample size | OS | DFS | Recurrence | Lymph node counts | Lymph node stations | Upstaging rate |
|---|---|---|---|---|---|---|---|---|---|
| Comparative analysis | |||||||||
| Hennon et al. (8) | 2020 | NCDB | 64,676 | NR | NR | NR | VATS (11.3) > RATS (10.9), P<0.01 | NR | VATS (11.2%) < RATS (11.7%), P<0.01 |
| Merritt et al. (9) | 2023 | NCDB | 20,834 | RATS (59.4%) < VATS (62.7%), P=NS | NR | NR | RATS (12.6) < VATS (12.9), P<0.001 | NR | RATS (11.79%) > VATS (11.49%), P=0.28 |
| Sesti et al. (10) | 2020 | SEER-Medicare | 818 | RATS (73.1%) > VATS (71.4%), P=0.37 | RATS (14.9%) < VATS (16.6%), P=0.64 | NR | NR | NR | NR |
| Shagabayeva et al. (11) | 2023 | NCDB | 9,304 | RATS (55.2%) < VATS (56%), P=0.95 | NR | NR | RATS [12] = VATS [12], P=0.23 | NR | RATS (14.7%) > VATS (12.9%), P=0.21 |
| Kneuertz et al. (12) | 2019 | 2 centers | 911 | NR | NR | NR | RATS (11.8) = VATS (11.8), P=0.95 | RATS (3.8) > VATS (3.6), P=0.001 | RATS (16.2%) > VATS (12.3%), P=0.03 |
| Yang et al. (4) | 2017 | NCDB | 470 | RATS (77.6%) > VATS (73.5%), P=0.1 | RATS (72.7%) > VATS (65.5%), P=0.047 | NR | NR | RATS [5] > VATS [3], P<0.001 | NR |
| Kent et al. (7) | 2023 | 21 centers | 6,646 | RATS (81%) > VATS (74%), P=0.001 | NR | RATS (15.9%) < VATS (19.2%), P=0.003 | NR | RATS (5.2) > VATS (4.5), P<0.001 | NR |
| Meta-analysis | |||||||||
| Aiolfi et al. (13) | 2021 | – | 34‡ | RATS (79.9%) > VATS (79.1%), P=NS | NR | NR | RATS (11.5) > VATS (10.4), P=NS | RATS (3.95) < VATS (4.56), P=NS | NR |
| Leitao et al. (6) | 2023 | – | 15‡ | RATS = VATS, HR =0.86, P=0.09 | RATS > VATS, HR =0.74, P=0.009 | NR | NR | NR | NR |
| Ma et al. (14) | 2021 | – | 18‡ | RATS = VATS, HR =1.02, P=0.88 | RATS = VATS, HR =1.03, P=0.89 | RATS > VATS, OR =0.51, P<0.001 | RATS > VATS, WMD =1.72, P=0.002 | RATS > VATS, WMD =0.51, P=0.005 | NR |
| Mao et al. (15) | 2021 | – | 18‡ | NR | NR | NR | RATS > VATS, SMD =0.308, P=0.001 | RATS = VATS, SMD =0.31, P=0.13 | NR |
| Wu et al. (16) | 2021 | – | 25‡ | RATS = VATS, HR =0.77, P=0.1 | RATS > VATS, HR =0.76, P=0.03 | NR | NR | NR | RATS = VATS, OR =0.89, P=0.68 |
| Guo et al. (17) | 2019 | – | 14‡ | NR | NR | NR | RATS = VATS, MD =0.87, P=0.39 | NR | NR |
| Liang et al. (18) | 2018 | – | 14‡ | NR | NR | NR | RATS [12] > VATS (10.6), P=0.38 | RATS [5] > VATS (4.3), P=0.26 | NR |
| O’Sullivan et al. (19) | 2019 | – | 13‡ | NR | NR | NR | VATS > RATS, SMD =−0.12, P<0.001 | NR | NR |
| Zhang et al. (20) | 2022 | – | 26‡ | RATS = VATS, OR =0.96, P=0.22 | RATS > VATS, OR =1.69, P=0.01 | RATS = VATS, OR =0.7, P=0.08 | RATS > VATS, OR =1.18, P<0.001 | RATS = VATS, MD =−0.15, P=0.73 | NR |
| Hu et al. (21) | 2019 | – | 20‡ | NR | NR | NR | RATS = VATS, MD =10.1, P=0.93 | RATS = VATS, MD =0.98, P=0.23 | NR |
| RCT | |||||||||
| Jin et al. (RVlob trial) (22) | 2022 | Single center† | 320 | NR | NR | NR | RATS [11] > VATS [10], P=0.02 | RATS [6] > VATS [5], P<0.001 | NR |
| Veronesi et al. (ROMAN study) (23) | 2021 | Multicenter† | 83 | NR | NR | NR | RATS (15.9) > VATS (10.2), P<0.001 | RATS [6] > VATS [4], P<0.001 | NR |
†, method; ‡, N studies included. VATS, video-assisted thorascopic surgery; OS, overall survival; DFS, disease-free survival; NCDB, National Cancer Database; NR, not reported; RATS, robotic-assisted thorascopic surgery; NS, not significant; SEER, Surveillance, Epidemiology, and End Results; WMD, weighted mean difference; SMD, standardized mean difference; RCT, randomized controlled trial.
There were 10 studies that reported OS, including 5 comparative analyses and 5 meta-analyses (4,6,7,9-11,13,14,16,20). Almost all of these studies (9 of the 10) observed no statistically significant difference in OS between VATS and robotic lobectomy. The exception was a comparative analysis by Kent et al. who aggregated data for the PORTaL study from clinical stage IA–IIIA non-small cell lung cancer (NSCLC) patients from 21 centers who underwent open versus robotic versus VATS lobectomy (7). They performed a propensity-matched analysis and found that open lobectomy had a 5-year OS of 85%, followed by robotic at 81%, and VATS at 74% (P=0.001). Furthermore, Cox multivariable analyses demonstrated that robotic lobectomy was associated with a significantly better OS as compared to VATS (HR =0.79, P=0.007). A multidisciplinary study by Leitao et al. found better OS with robotic lobectomy versus open lobectomy (HR =0.93 95% CI: 0.87–1, P=0.04), but this statistically significant benefit was not held true when comparing robotic to VATS approach (6). Neither of the 2 RCTs included in this review reported OS for various reasons. The ROMAN study failed to accrue and was thus closed prematurely, but their primary outcome focused on conversion rate and early complications, as opposed to long-term oncologic results (23). On the other hand, the RVlob trial was recently published in 2022, and long-term survival data has not matured but will be highly anticipated (22,24).
There were 6 studies that reported DFS, including 2 comparative analysis and 4 meta-analyses (4,6,10,14,16,20). Of these studies, 2 did not find any statistically significant differences in DFS between VATS and robotic lobectomy (9,10). However, there were 3 meta-analyses that reported a significant advantage with a robotic approach, including the study by Leitao et al. (HR =0.74, 95% CI: 0.59–0.93, P=0.009), Wu et al. (HR =0.76, 95% CI: 0.59–0.97, P=0.03), and Zhang et al. (OR =1.69, 95% CI: 1.11–2.57, P=0.01; I2=23%) (6,16,20).
There were 3 studies that reported recurrence rates, including 1 comparative analysis and 2 meta-analyses (7,14,21). Of these studies, 2 of the 3 reported a significantly improved recurrence rates with robotic lobectomy compared to VATS, whereas the other study reported no significant difference. One of the studies that showed better outcomes was the PORTaL study reported by Kent et al. who found statistically lower recurrence rates in the robotic cohort than VATS (15.9% versus 19.2%, P=0.003) (7). Similarly, Ma et al. found lower recurrence rates with robotic lobectomy versus VATS (OR =0.51, 95% CI: 0.36–0.72, P<0.001), and this held true on subgroup analysis and sensitivity analysis including only the highest-quality studies (14).
There were 14 studies that reported lymph node counts, including 4 comparative analyses, 8 meta-analyses, and 2 RCTs. Of these, half of the studies (6 of the 14) reported no significant differences in extent of lymph node harvest between VATS and robotic lobectomy (11-13,17,18,21). Of the remaining 8 studies that did find significant differences, the results were more favorable for robotic lobectomy in 5 of 8 studies. These studies including 3 meta-analyses and 2 RCTs, and demonstrated better nodal harvests with robotic lobectomy (14,15,20,22,23). The RVlob trial showed a median of 11 versus 10 nodes sampled (P=0.02), and the ROMAN study showed a median of 7 versus 4 hilar nodes and 7 versus 5 mediastinal nodes retrieved (P=0.0001) for robotic lobectomy as compared to VATS (22,23). However, in 3 of the 8 studies, including 2 comparative analyses and 1 meta-analysis, VATS was found to have higher lymph node counts (4,8,19).
There were 11 studies that reported lymph node stations sampled, including 3 comparative analyses, 6 meta-analyses, and 2 RCTs. Six of these studies demonstrated superiority with robotic lobectomy, while the other 5 studies reported no significance. Of the 2 meta-analyses that showed favorable results for robotic lobectomy, Kneuertz et al. demonstrated slightly more stations sampled with the robotic approach (3.8 versus 2.6, P=0.001) (12), while Ma et al. reported a significant advantage with robotic lobectomy (WMD =0.51, 95% CI: 0.15–0.86, P=0.005) (14). Of the 2 RCTS that showed significant differences, the RVlob trial found that robotic lobectomy sampled a median of 6 versus 5 stations (P<0.001) (22) and the ROMAN study similarly reported a median of 6 versus 4 stations (P=0.0002).
There were 5 studies that reported upstaging rate, including 4 comparative analyses and 1 meta-analysis (8,9,11,12,16). Only 2 of the 5 studies showed a significant difference between approaches and the results are equivocal. The comparative analysis by Hennon et al. favored VATS, showing that robotic lobectomy had an upstaging rate of 11.2% versus 11.7% for VATS (P<0.01) (8), whereas the analysis by Kneuertz et al. favored the robotic approach, citing an upstaging rate of 16.2% versus 12.3% for VATS (P=0.03) (12).
Discussion
Key findings
The use of robotic surgery in thoracic surgery has shown to be safe and effective (25-27), with consistent reports of improved perioperative and short-term outcomes such as hospital length of stay, postoperative complications, and 30-day mortality as compared to both open and VATS (1-3,26). As the procedure has matured, current research focus has shifted to evaluating longer term outcomes, specifically OS, DFS, and recurrence rates in lung cancer. Nevertheless, in this review of the literature, it is apparent that evidence is still sparse. There is a lack of high-quality RCTs or large-sized studies to comparatively analyze RATS versus VATS in unbiased, head-to-head oncologic outcomes, so we must rely on well-executed comparative analyses that aggregate modern data to shed some light on the clinically significant risks and benefits of this ongoing practice change.
Strengths and limitations
This scoping review is unique such that it focuses on oncologic specific outcomes, namely, OS, DFS, and recurrence rates, which prior systematic reviews have not done. Nevertheless, we also acknowledge the limitations with this study, namely the lack of high quality Level I and II evidence that is available on this topic. In addition, some of the studies included did not stratify patients according to preoperative characteristics such as pulmonary function and comorbidity status, and instead simply stratified patients into the various surgical approaches. Lastly, among the meta-analyses included in the results, there were 7 out of 69 studies that were duplicated in the statistical analysis. This may have led to bias towards the conclusions of these particular 7 studies which are unaccounted for.
Comparison with similar researches
Many of the published literature reviews assessing robotic versus VATS lobectomy use short-term perioperative variables as the main outcome. However, the safety and feasibility of RATS in lung cancer treatment has been validated, thus our review provides a novel amalgamation of current studies which continue to highlight the gaps in this field.
Explanation of findings
In our analysis, there was little evidence to show any difference in OS between RATS and VATS. OS is a problematic endpoint since patients may die of causes other than lung cancer recurrence or metastasis. Given that the majority of patients are diagnosed with lung cancer after age 65, and subsequently undergoing surgery in a later stage in life, OS may reflect too much hetereogeneity to be a suitable endpoint for oncologic treatment efficacy (27).
In contrast, there is some evidence to suggest DFS is superior for RATS compared to VATS. This endpoint is more specific to cancer outcomes and may be a better metric of oncologic efficacy. The reason this is observed could be partially due to the superior lymph node harvest and yields, leading to more accurate pathologic staging, and better subsequent adjuvant care, which has been shown in several publications for RATS. The purported advantage of robotic technology in this task is due to better reach and access, such as lymph node station 7, as well as better visualization and precision for more difficult nodes such as the hilar stations.
The importance of lymph node dissection on oncologic quality is reflected in society guidelines. For example, the American College of Surgeons Commission on Cancer recently updated their recommendations from a count-based to a station-based approach, now requiring at least 1 hilar and 3 mediastinal lymph node stations to be performed during lung resection (28). This change is important for future studies to take into consideration, as published results are already showing that adherence to station-based sampling is significantly associated with DFS, whereas count-based sampling is not (29). It is hoped that having more standardized lymphadenectomy requirements will lead to higher quality resections and reduce variability in oncologic outcomes. This could also help elucidate lymph node upstaging rates in each of the surgical approaches. With the national average rate of nodal upstaging previously cited to be only 10–15% (30), larger cohorts of patients are needed to sufficiently power this outcome. Moreover, if lymph node retrieval rates are inconsistent and include low yields, then the upstaging rate may not be accurately assessed. While one of the included studies found a higher rate of nodal upstaging with robotic lobectomy, the cumulative evidence does not achieve statistical significance. Future multi-institutional trials from high volume robotic surgery centers with standardized lymph node dissection approaches will be vital to assess this surgical quality measure. It is apparent that individual surgeon philosophy about the aggressiveness of lymph node dissection can also influence outcomes, so using the same surgeons in the comparisons will be optimal.
In addition, limited data is available regarding adjuvant treatment. This highlights an important confounding variable that is unaccounted for in patient selection when considering robotic versus VATS lobectomy. Studies often do not report the number of patients who completed treatment versus those who had modifications or could not complete adjuvant treatment, so true head-to-head comparisons would need to focus only on early stage lung cancer where patients generally do not receive adjuvant therapy. Management of stage 2 and 3 lung cancer is so variable worldwide, and is often based on ever-changing variables such as year of diagnosis and patient tolerance. Therefore, surrogate markers like lymph node harvest rates and R0 resection rates play a significant role in elucidating the differences between robotic versus VATS lobectomy.
Implications and actions needed
While many surgeons posit that only RCTs can provide sufficient data to assess outcomes for a new technology such as robotic surgery, turning that aspiration into reality is a challenging task. In the United States, adoption of robotic technology in 2020 was nearly 50% for lobectomy and >50% for segmentectomy, and overall has become significantly more popular than VATS (31). Rapid development of surgeon preference leading to more comfort and higher skill level tailored to one of the two minimally invasive surgical approaches contributes to the difficulty in completing RCTs with equipoise at the individual surgeon level. To date, four RCTs comparing RATS to VATS have been reported, and only one has sufficient numbers and recruitment (22,23,32,33). Three of the RCTs were not optimal and reflect the extreme difficulty in conducting such studies: one was a multi-surgeon, multi-center study that closed prematurely due to poor accrual, and the other two RCTs were small, single surgeon studies (22,23,32). Furthermore, none of these 3 studies reported long-term cancer outcomes to date, and 2 of them reported lymph node yield data which were analyzed in the present review article. The RAVAL study led by Hanna et al. recently reported in abstract form superior short-term outcomes (including better lymph node yield with RATS) from 186 randomized patients showing improved perioperative outcomes and patient-reported quality of life indicators of RATS compared to VATS, but the long-term cancer outcomes await greater recruitment and follow-up, and is still ongoing (33,34).
Conclusions
Robotic surgery in thoracic oncologic surgery is safe and effective when assessing both short-and long-term outcomes. The published data to date indicate some benefit of DFS with RATS compared to VATS. Lymph node dissection yield, an indicator of oncologic quality, appears to be improved with RATS in some but not all studies. Future RCT data will be welcome to further elucidate these differences.
Acknowledgments
The authors would like to thank the library staff and resources at Intuitive Surgical.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Michael Shackcloth and Amer Harky) for the series “Lung Cancer Surgery” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA-ScR reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-23-26/rc
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-23-26/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-23-26/coif). The series “Lung Cancer Surgery” was commissioned by the editorial office without any funding or sponsorship. DSO is a part-time medical advisor to Intuitive Surgical. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg 2011;128:305-10. [Crossref] [PubMed]
- Leitao MM Jr, Kreaden US, Laudone V, et al. The RECOURSE Study: Long-term Oncologic Outcomes Associated With Robotically Assisted Minimally Invasive Procedures for Endometrial, Cervical, Colorectal, Lung, or Prostate Cancer: A Systematic Review and Meta-analysis. Ann Surg 2023;277:387-96. [Crossref] [PubMed]
- Kent MS, Hartwig MG, Vallières E, et al. Pulmonary Open, Robotic and Thoracoscopic Lobectomy (PORTaL) Study: Survival Analysis of 6,646 Cases. Ann Surg 2023; Epub ahead of print. [Crossref] [PubMed]
- Hennon MW, DeGraaff LH, Groman A, et al. The association of nodal upstaging with surgical approach and its impact on long-term survival after resection of non-small-cell lung cancer. Eur J Cardiothorac Surg 2020;57:888-95. [Crossref] [PubMed]
- Merritt RE, Abdel-Rasoul M, D'Souza DM, et al. Lymph Node Upstaging for Robotic, Thoracoscopic, and Open Lobectomy for Stage T2-3N0 Lung Cancer. Ann Thorac Surg 2023;115:175-82. [Crossref] [PubMed]
- Sesti J, Langan RC, Bell J, et al. A Comparative Analysis of Long-Term Survival of Robotic Versus Thoracoscopic Lobectomy. Ann Thorac Surg 2020;110:1139-46. [Crossref] [PubMed]
- Shagabayeva L, Fu B, Panda N, et al. Open, Video- and Robot-Assisted Thoracoscopic Lobectomy for Stage II-IIIA Non-Small Cell Lung Cancer. Ann Thorac Surg 2023;115:184-90. [Crossref] [PubMed]
- Kneuertz PJ, Cheufou DH, D'Souza DM, et al. Propensity-score adjusted comparison of pathologic nodal upstaging by robotic, video-assisted thoracoscopic, and open lobectomy for non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:1457-1466.e2. [Crossref] [PubMed]
- Aiolfi A, Nosotti M, Micheletto G, et al. Pulmonary lobectomy for cancer: Systematic review and network meta-analysis comparing open, video-assisted thoracic surgery, and robotic approach. Surgery 2021;169:436-46. [Crossref] [PubMed]
- Ma J, Li X, Zhao S, et al. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non-small cell lung cancer: a meta-analysis. BMC Cancer 2021;21:498. [Crossref] [PubMed]
- Mao J, Tang Z, Mi Y, et al. Robotic and video-assisted lobectomy/segmentectomy for non-small cell lung cancer have similar perioperative outcomes: a systematic review and meta-analysis. Transl Cancer Res 2021;10:3883-93. [Crossref] [PubMed]
- Wu H, Jin R, Yang S, et al. Long-term and short-term outcomes of robot- versus video-assisted anatomic lung resection in lung cancer: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2021;59:732-40. [Crossref] [PubMed]
- Guo F, Ma D, Li S. Compare the prognosis of Da Vinci robot-assisted thoracic surgery (RATS) with video-assisted thoracic surgery (VATS) for non-small cell lung cancer: A Meta-analysis. Medicine (Baltimore) 2019;98:e17089. [Crossref] [PubMed]
- Liang H, Liang W, Zhao L, et al. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann Surg 2018;268:254-9. [Crossref] [PubMed]
- O'Sullivan KE, Kreaden US, Hebert AE, et al. A systematic review and meta-analysis of robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. Interact Cardiovasc Thorac Surg 2019;28:526-34. [Crossref] [PubMed]
- Zhang J, Feng Q, Huang Y, et al. Updated Evaluation of Robotic- and Video-Assisted Thoracoscopic Lobectomy or Segmentectomy for Lung Cancer: A Systematic Review and Meta-Analysis. Front Oncol 2022;12:853530. [Crossref] [PubMed]
- Hu X, Wang M. Efficacy and Safety of Robot-assisted Thoracic Surgery (RATS) Compare with Video-assisted Thoracoscopic Surgery (VATS) for Lung Lobectomy in Patients with Non-small Cell Lung Cancer. Comb Chem High Throughput Screen 2019;22:169-78. [Crossref] [PubMed]
- Jin R, Zheng Y, Yuan Y, et al. Robotic-assisted Versus Video-assisted Thoracoscopic Lobectomy: Short-term Results of a Randomized Clinical Trial (RVlob Trial). Ann Surg 2022;275:295-302. [Crossref] [PubMed]
- Veronesi G, Abbas AE, Muriana P, et al. Perioperative Outcome of Robotic Approach Versus Manual Videothoracoscopic Major Resection in Patients Affected by Early Lung Cancer: Results of a Randomized Multicentric Study (ROMAN Study). Front Oncol 2021;11:726408. [Crossref] [PubMed]
- Kneuertz PJ, D'Souza DM, Richardson M, et al. Long-Term Oncologic Outcomes After Robotic Lobectomy for Early-stage Non-Small-cell Lung Cancer Versus Video-assisted Thoracoscopic and Open Thoracotomy Approach. Clin Lung Cancer 2020;21:214-224.e2. [Crossref] [PubMed]
- Oh DS, Cho I, Karamian B, et al. Early adoption of robotic pulmonary lobectomy: feasibility and initial outcomes. Am Surg 2013;79:1075-80. [Crossref] [PubMed]
- Yang CF, Sun Z, Speicher PJ, et al. Use and Outcomes of Minimally Invasive Lobectomy for Stage I Non-Small Cell Lung Cancer in the National Cancer Data Base. Ann Thorac Surg 2016;101:1037-42. [Crossref] [PubMed]
- American Cancer Society. Facts & Figures 2023. American Cancer Society. Atlanta, GA; 2023.
- National Comprehensive Cancer Network. NCCN Guidelines in Oncology: non-small cell lung cancer, Version 6. 2020.
- Heiden BT, Eaton DB Jr, Chang SH, et al. Assessment of Updated Commission on Cancer Guidelines for Intraoperative Lymph Node Sampling in Early Stage NSCLC. J Thorac Oncol 2022;17:1287-96. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 353. [Crossref] [PubMed]
- Servais EL, Blasberg JD, Brown LM, et al. The Society of Thoracic Surgeons General Thoracic Surgery Database: 2022 Update on Outcomes and Research. Ann Thorac Surg 2023;115:43-9. [Crossref] [PubMed]
- Terra RM, Araujo PHXN, Lauricella LL, et al. A Brazilian randomized study: Robotic-Assisted vs. Video-assisted lung lobectomy Outcomes (BRAVO trial). J Bras Pneumol 2022;48:e20210464. [Crossref] [PubMed]
- Patel YS, Hanna WC, Fahim C, et al. RAVAL trial: Protocol of an international, multi-centered, blinded, randomized controlled trial comparing robotic-assisted versus video-assisted lobectomy for early-stage lung cancer. PLoS One 2022;17:e0261767. [Crossref] [PubMed]
- Hanna WC, Patel YS, Baste JM, et al. Robotic-Assisted Lobectomy for Early-Stage Lung Cancer Provides Better Patient-Reported Quality of Life Compared to Video-Assisted Lobectomy: Early Results of the RAVAL Trial. [cited 2023 Mar 13]; Available online: https://www.aats.org/resources/2966
Cite this article as: Wong LY, Oh DS. Scoping review on long term oncologic outcomes in robotic-assisted lobectomy. Video-assist Thorac Surg 2023;8:28.