
Anatomy of the lung revisited by 3D-CT imaging
Introduction
The anatomy of the lung has been studied in detail since the 1930’s with an international nomenclature proposed in 1950 (1). Knowledge of lung segments has been periodically revised, mostly based on input from cadaveric studies and surgical findings (2-5). This led to a textbook presented by Yamashita in 1978 which today remains widely used and a key reference in many publications (5). However, information collected from cadavers has drawbacks including postmortem modifications or limitations in analysis due to difficulties procuring cases. Since these initial cadaveric and surgical studies, non-invasive imaging technology has flourished enabling analyses from a radiological perspective. In particular, computed tomography (CT) and three-dimensional (3D) imaging has made it possible to reconstruct the anatomy of the lung allowing for a more intuitive understanding of the spatial relationships between structures (6). 3D-CT imaging involves postprocessing of the entire multidetector CT data set to generate 3D volume-rendered images, allowing for visualization and manipulation of objects represented as sample data in 3D. The adoption of 3D-CT imaging has led to a variety of analyses led not only by anatomists but also by surgeons and radiologists. Thoracic surgeons also became actively engaged in anatomical analysis using 3D imaging, partly driven by the surgical need to better understand the anatomy of the lung (7-12). We will review here key features of 3D-CT imaging of the lung and present representative anatomical studies based on 3D-CT images.
Features of 3D-CT Imaging
A major advantage of 3D-CT imaging lies in its ability to allow for better recognition of anatomical structures compared to two-dimensional (2D) images (Table 1). Although conventional CT images do include information necessary to analyze anatomy, they are not always sufficient to fully perceive the spatial relationships between anatomical structures. A trained physician could recreate anatomical structures in his or her mind (13), but the technique requires skill and is time consuming. 3D-CT images enable surgeons to quickly and more intuitively recognize the anatomy and associated anomalies (14). Also, images can be shared for educational purposes or used for pre- and intra-operative simulation (10).
Table 1
| • Better recognition of anatomical structures compared to two-dimensional images |
| • Quick and intuitive recognition of the anatomy and anomalies |
| • Pre- and intra-operative simulation |
| • Improved surgical safety |
| • Enhanced educational utility |
| • New types of analyses involving lung volume or lung parenchyma |
| • Large-scale studies with short study times (>1,000 cases) |
3D, three-dimensional; CT, computed tomography.
Another benefit of 3D-CT imaging is the associated improvement in surgical safety. With the increasing number of segmentectomies being performed, a detailed understanding of each patient’s unique anatomy, which includes the spatial relationship between bronchial, vascular, and parenchymal structures, is of increasing importance. In 2011, Oizumi et al. presented the usefulness of preoperative 3D-CT imaging in thoracoscopic segmentectomy and reported a 98% success rate (8). Many others have also reported the benefits of 3D-CT imaging in lung surgery (15). Surgeons performing segmentectomy are also more likely to be faced with segmental variations, and some commonly encountered variations have been analyzed in more detail, such as the subsuperior segment (16-18). Accordingly, an understanding of segmental anatomy and associated anatomic variants is essential for segmentectomy (Figure 1). However, a basic knowledge of anatomical variations is also beneficial when performing a routine lobectomy. We should always bear in mind the possibility of encountering common variations, such as an anomalous V2, a mediastinal lingular artery, or a lingular vein draining into the inferior pulmonary vein, each of which may result in surgical complications should they fail to be appreciated.
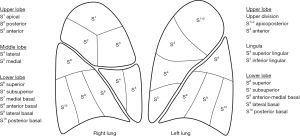
The use of 3D-CT imaging furthermore allows for new types of analysis such as the relationship between lung volume and segmental anatomy (19) or the spatial relationship between intersegmental planes and intersegmental veins (20). Mimae et al. reported that the main root of the intersegmental vein (V3a+b) between the upper and lingular divisions was always located in the upper division, whereas the root of the intersegmental vein (V6b+c) between S6 and basal segments was always located in the basal segment; it is important to know that intersegmental veins are not always located on the intersegmental plane when dividing the lung parenchyma along these intersegmental veins (20).
Also, several 3D-CT imaging studies have enrolled a large number of patients within a very short amount of time, some including more than 1,000 cases and others occasionally exceeding 5,000 cases (17,21-23). Such large-scale analyses would be difficult in a cadaveric study. Furthermore, classifying all cases into diverse anatomical categories with 2D images alone would also be an immensely complicated task. Accordingly, the volume, speed, and rigor at which complex anatomic studies may be conducted and analyzed using 3D imaging provides distinct advantages over studies using cadaveric specimens or 2D images alone.
General anatomy of the lung
In 2010, Akiba et al. analyzed variations of the pulmonary vein using 3D-CT images (Table 2) (24). They reported that most patients had the expected anatomy (98% of the left side and 86% of the right side). Common ostia were more frequent on the left side than on the right side (33% vs. 13%); the middle lobe drained directly into the left atrium or inferior pulmonary vein in 11% of patients; and the right inferior pulmonary vein often divided immediately at the root in 23% of patients. Fourdrain et al. also analyzed variations of the pulmonary arteries and veins (25,26). For pulmonary veins, 36% of patients had variations, and variations were more frequent on the right side than on the left side. The most frequent right-sided variation was the existence of three separate pulmonary veins, whereas the most frequent left-sided variation was the existence of a single pulmonary vein. Shiina et al. also analyzed variations of the pulmonary vein in the right upper lobe (RUL), right middle lobe (RML), right lower lobe (RLL), and left upper lobe (LUL) and reported that the incidence of variations ranged from 2.6% to 15.3%, but found no variants in the left lower lobe (LLL). They emphasized the importance of variations that could be critical during lung resection, such as anomalous V2, V6, RML veins, and lingular veins (27).
Table 2
| Anatomical features | Authors (ref.) | Bronchus | Pulmonary artery | Pulmonary vein |
|---|---|---|---|---|
| Lung in general | Akiba et al. (24) | N/A | N/A | Common ostia |
| Variations of venous drainage | ||||
| Fourdrain et al. (25,26) | N/A | Number of branches, frequency, and variations in all lobes | Right side: 6 types | |
| Left side: 4 types | ||||
| Shiina et al. (27) | N/A | N/A | Anomalous drainage of right V2/V3/V6 and left lingular veins | |
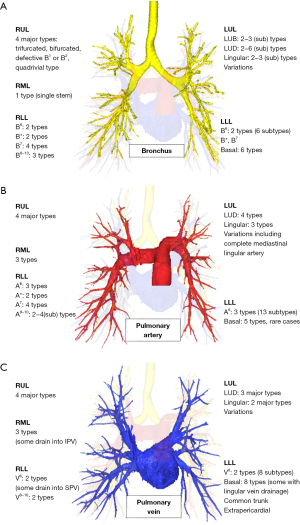
| RUL | Nagashima et al. (28) | 4 major types: trifurcated, bifurcated, defective B1 or B2, quadrivial type | 4 major types | 4 major types |
| RML | Nagashima et al. (29) | 1 type (single stem) | 3 types | 3 types (some drain into IPV) |
| RLL | Nagashima et al. (29) | B6/B*/B7/B8-10: 2/2/4/3 types | A6/A*/A7: 3/2/4 types | V6: 2 major types (some drain into SPV) |
| A8-10: 2 major types (4 subtypes) | V8-10: 2 major types (5 subtypes) | |||
| LUL | Deng et al. (30) | 2 types (8 subtypes) | 3 types (10 subtypes) of variations | 2 types (5 subtypes) of variations |
| Fan et al. (31) | LUD: 4 types | LUD: 4 types | LUD: 3 types | |
| Lingula: 3 types | Lingula: 3 types | Lingula: 2 types | ||
| Uncommon variations | Uncommon variations | Uncommon variations | ||
| Maki et al. (32) | LUB: 2 major types | 2 types according to lingular artery (15 subtypes) | LUD: 3 major types (13 subtypes) | |
| LUD: 2 major types (4 subtypes) | Complete mediastinal lingular artery and rare variations | Lingula: 2 major types (8 subtypes) | ||
| lingula: 2 types (1 subtype) | ||||
| rare variations | ||||
| He et al. (33) | LUB: 2 types (3 subtypes) | N/A | N/A | |
| LUD: 2 types (6 subtypes) | ||||
| lingula: 2 types (3 subtypes) | ||||
| LLL | Maki et al. (34) | B6: 2 types (6 subtypes) | A6: 3 types (13 subtypes) | V6: 2 types (8 subtypes) |
| Basal bronchus: 6 types | Basal artery: 5 types, rare cases | Basal vein: 8 types and lingular vein draining into the LLV | ||
| B*, B7 | Common trunk, extrapericardial | |||
| Interlobar vessels | Wang et al., Xu et al., Murota et al. (35-37) | N/A | Right side: 4 types (15 subtypes) | Right side: 2 major types (30 subtypes) |
| Left side: 7 types (85 subtypes) |
For clarity, the number of types and subtypes have been simplified in some cases and the reader should refer to the original manuscript for a detailed classification. 3D, three-dimensional; CT, computed tomography; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; LUD, left upper division; LUB, left upper bronchus; PV, pulmonary vein; SPV, superior pulmonary vein; IPV, inferior pulmonary vein; LLV, left lower vein; N/A, not assessed; ref., references.
Anatomy of the right lobes
In 2015, based on 3D-CT angiography and bronchiography (3D-CTAB), we analyzed the anatomical variations of RUL in more detail and compared data with those in previous cadaveric studies (Table 2, Figures 2,3) (28). Although the incidence of variations in pulmonary arteries was similar, there were differences in the incidence of variations in veins and bronchi, such as the B1- or B2-defective patterns. Zhang et al. later studied the B1-defective type in more detail, and additionally analyzed variations in vascular patterns (38). Based on anatomical data, we further created a simplified model of segmental anatomy to guide surgeons while performing segmentectomies (39,40). Our aim was to classify the wide-variety of segmental anatomy into several specific patterns, allowing surgeons to perform segmentectomies with a pattern-based approach. Zhang et al. also created a simplified anatomical model for the left upper division which can be considered to be the counterpart of the RUL (41).
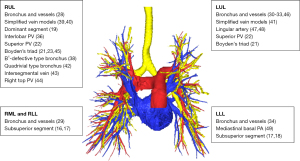
Interestingly, some have studied the RUL anatomy by analyzing anatomical structures bilaterally or by taking into account lung volume as a factor for classification. Wang et al. compared the superior pulmonary veins bilaterally and proposed a uniform classification that could be applicable for both upper lobes, that is, classifying the veins into central, semi-central, and non-central types (22). Chen et al. included segmental lung volume analyzed by 3D imaging as a factor to determine the “dominant pulmonary segment” of the RUL and subsequently determined whether segmental lung volume could be correlated to anatomical variations (19). Other studies analyzed more specific features of the RUL such as the quadrivial pattern bronchus (42), the V2a intersegmental vein (43), the right top pulmonary vein (44), or Boyden’s triad (21,23,45).
In 2017, we subsequently reported on the segmental anatomy of the RML and RLL (Table 2, Figure 3) (29). Pulmonary bronchi and vessels of the RML and S6 were classified according to the number of stems. Bronchi and pulmonary arteries of S7 and basal segments were classified by branching patterns. Also, the subsuperior segment (or S*), which is an independent segment between S6 and S10, was identified in 20.4% of cases. Studies further analyzed the subsuperior segment in more detail, not only on the right side but also bilaterally (16-18).
Anatomy of the left lobes
Between 2020 and 2022, several studies evaluated the LUL anatomy, each using slightly different classification systems (Table 2, Figure 3) (30-33). Isaka et al. also analyzed the relationship between branching patterns of bronchi, arteries, and veins, finding a significant correlation between arterial and bronchial branching patterns as well as between arterial and venous branching patterns (46). As previously mentioned, Zhang et al. classified the veins of the left upper division into simplified models that can be used during segmentectomies (41). Additional studies also focused on the lingular artery, with one study suggesting that the mediastinal lingular artery might originate from the variation of B3, and that the presence of a mediastinal lingual artery also influences the venous pattern of the left upper division (47,48).
In 2020, Maki et al. reported the anatomy of the LLL (Table 2, Figure 3) (34). They also reported rare variations such as B7 with independent branching from the basal bronchi; subsuperior bronchus (B*); or an extrapericardial common trunk of the left pulmonary veins. Liu et al. proposed a classification for the mediastinal basal artery, which is a pulmonary artery that branches from the proximal part of the left pulmonary artery between the left main bronchus and the left superior pulmonary vein, proceeding directly into the lower lobe (49). The study by Maki et al. also included one case of a mediastinal basal artery that branched within the pericardium (34).
Interlobar vessels
Some studies have analyzed the variations of interlobar vessels (Table 2). Information on interlobar vessels would be important for surgeons when identifying these vessels during anatomical lung resection or when dissecting the lung fissure in patients with incomplete lobulation. Wang et al. classified the right interlobar arteries according to the order and number of branches of the RML artery and A6 (35). Xu et al. classified the general morphology of the right interlobar veins and reported that interlobar veins hidden by an incomplete upper oblique fissure were most vulnerable to accidental injury; a diameter larger than 2.4 mm for the oblique fissure interlobar vein type or less than 2 mm for the mediastinal interlobar vein type was also associated with a higher risk of injury (36). Murota et al. also classified left-side interlobar arteries (37).
Limitations of 3D imaging and development of 3D imaging software
Despite the advantages and contribution to our current understanding of lung anatomy as detailed above, current 3D-CT imaging has its limitations. For example, small blood vessels that are visible on conventional 2D images may not always be reconstructed in 3D-CT images. In our previous study, small vessels with a diameter of less than 1.5 mm were missed on 3D-CT images when compared to intraoperative views (28). For a better identification of small anatomical structures, observing both 3D-CT images and thin-section CT images is equally important. There will always be some inherent difference between 2D images, 3D-CT reconstructed images, and intraoperative views. Vessels that were preoperatively overlooked by 2D or 3D images might only be recognized intraoperatively. Therefore, feedback from actual intraoperative findings is important to further refine 3D-CT reconstruction methods. Use of 3D-CT images could also be limited by other factors including limited availability of 3D reconstruction software; inadequate conditions during CT examination; and the presence of tumors or atelectasis obstructing bronchi, impeding vascular flow, or obscuring peripheral anatomy. To overcome these limitations, numerous imaging software platforms have been developed and optimized, including Ziostation 2 (Ziosoft Inc.), REVORAS (Ziosoft Inc.), Synapse Vincent (Fujifilm), IQQA-Lung (EDDA Technology), Deepinsight platform (Neusoft Group Ltd.), Mimics software (Materialise), PV-iCAS (PVmed), and the list continues to grow (19,21,28,33,44,45,48). Until now, the prerequisite for 3D imaging of pulmonary vessels was the availability of contrast-enhanced CT images. However, 3D imaging software now allows for 3D reconstruction of pulmonary vessels from non-enhanced CT data (50-53).
Conclusions
We reviewed here key features of 3D-CT imaging of the lung and presented results from representative anatomical studies. Such studies have led to new or modified classification systems, shed light on lung anatomy from a useful surgical viewpoint, and enabled us to analyze lung anatomy with a focus on particular anatomical features. 3D-CT images also allow for enhanced pre- and intra-operative simulation, improved surgical safety, enhanced educational utility, and the capacity to perform large-scale anatomical studies in shorter time frames. A decade has passed since the initial reports on 3D-CT image-guided lung resection (54-59) and 3D-CT imaging has become widely implemented with results of prospective multicenter studies now being reported (60). The current surge of 3D-CT imaging analysis shows that the field is still growing, with the technology continuing to improve and now even being combined with virtual reality and artificial intelligence (61,62). Future studies using these new and innovative methodologies will continue to refine our understanding of lung anatomy while enhancing our ability to perform safe and effective surgical resections.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Video-Assisted Thoracic Surgery for the series “Simulation and Navigation Techniques in VATS/RATS”. The article has undergone external peer review.
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-23-21/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-23-21/coif). The series “Simulation and Navigation Techniques in VATS/RATS” was commissioned by the editorial office without any funding or sponsorship. SN served as the unpaid Guest Editor of the series. HI served as the unpaid Guest Editor of the series and serves as the unpaid Associate Editor-in-Chief of Video-Assisted Thoracic Surgery from December 2022 to November 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- NOMENCLATURE of broncho-pulmonary anatomy; an international nomenclature accepted by the Thoracic Society. Thorax 1950;5:222-8. [Crossref] [PubMed]
- Cory RA, Valentine EJ. Varying patterns of the lobar branches of the pulmonary artery. A study of 524 lungs and lobes seen at operation of 426 patients. Thorax 1959;14:267-80. [Crossref] [PubMed]
- Boyden EA, Scannell JG. An analysis of variations in the bronchovascular pattern of the right upper lobe of 50 lungs. Am J Anat 1948;82:27-73. [Crossref] [PubMed]
- Boyden EA, Hartmann JF. An analysis of variations in the bronchopulmonary segments of the left upper lobes of fifty lungs. Am J Anat 1946;79:321-60. [Crossref] [PubMed]
- Yamashita H. Roentgenologic anatomy of the lung. Igaku-Shoin; 1978:389.
- Akiba T. Utility of three-dimensional computed tomography in general thoracic surgery. Gen Thorac Cardiovasc Surg 2013;61:676-84. [Crossref] [PubMed]
- Oizumi H, Endoh M, Takeda S, et al. Anatomical lung segmentectomy simulated by computed tomographic angiography. Ann Thorac Surg 2010;90:1382-3. [Crossref] [PubMed]
- Oizumi H, Kanauchi N, Kato H, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011;141:678-82. [Crossref] [PubMed]
- Kanzaki M, Maeda H, Wachi N, et al. Complete video-assisted thoracoscopic multi-subsegmentectomy based on patients’ specific virtual 3-D pulmonary models. Asian J Endosc Surg 2013;6:110-5. [Crossref] [PubMed]
- Chen-Yoshikawa TF, Date H. Update on three-dimensional image reconstruction for preoperative simulation in thoracic surgery. J Thorac Dis 2016;8:S295-301. [PubMed]
- Shimizu K, Nakazawa S, Nagashima T, et al. 3D-CT anatomy for VATS segmentectomy. J Vis Surg 2017;3:88. [Crossref] [PubMed]
- Nakazawa S, Shimizu K, Mogi A, et al. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg 2018;66:81-90. [Crossref] [PubMed]
- Wang R, Zhang Y, Hu Q, et al. Identification of the segmental structures of the right upper lobe of the lung using non-enhanced thin-slice CT. J Thorac Dis 2020;12:1639-44. [Crossref] [PubMed]
- Bhakhri K, Hyde ER, Mak SM, et al. Surgeon knowledge of the pulmonary arterial system and surgical plan confidence is improved by interactive virtual 3D-CT models of lung cancer patient anatomies. Front Surg 2021;8:652428. [Crossref] [PubMed]
- Vervoorn MT, Wulfse M, Mohamed Hoesein FAA, et al. Application of three-dimensional computed tomography imaging and reconstructive techniques in lung surgery: A mini-review. Front Surg 2022;9:1079857. [Crossref] [PubMed]
- Shimizu K, Mogi A, Yajima T, et al. Thoracoscopic subsuperior segment segmentectomy. Ann Thorac Surg 2017;104:e407-10. [Crossref] [PubMed]
- Zhou D, Gao Y, Wang H, et al. Prevalence and anatomical characteristics of subsuperior segment in lung lower lobe. J Thorac Cardiovasc Surg 2022;S0022-5223(22)00411-1.
- Maki R, Miyajima M, Ogura K, et al. Anatomy of the left subsuperior segment for segmentectomy. Surg Today 2022;52:1054-62. [Crossref] [PubMed]
- Chen ZH, Chu XP, Zhang JT, et al. The regularity of anatomical variations of dominant pulmonary segments in the right upper lobe. Thorac Cancer 2023;14:462-9. [Crossref] [PubMed]
- Mimae T, Miyata Y, Kumada T, et al. The intersegmental pulmonary vein is not always located on the intersegmental plane of the lung: Evaluation with 3-dimensional volume-rendering image reconstruction. JTCVS Tech 2022;16:132-8. [Crossref] [PubMed]
- Zhang M, Sun WJ, Wu QC, et al. Boyden’s triad in the left lung: an interesting phenomenon. Interact Cardiovasc Thorac Surg 2022;35:ivac082. [Crossref] [PubMed]
- Wang J, Lin H, Bian C, et al. A modified system for classifying the bilateral superior pulmonary veins using three-dimensional computed tomography bronchography and angiography images. J Thorac Dis 2021;13:5933-41. [Crossref] [PubMed]
- Zhang M, Mao N, Wu Q, et al. Boyden’s triad: the past, present and future. Interact Cardiovasc Thorac Surg 2022;34:590-6. [Crossref] [PubMed]
- Akiba T, Marushima H, Odaka M, et al. Pulmonary vein analysis using three-dimensional computed tomography angiography for thoracic surgery. Gen Thorac Cardiovasc Surg 2010;58:331-5. [Crossref] [PubMed]
- Fourdrain A, De Dominicis F, Bensussan M, et al. Three-dimensional computed tomography angiography of the pulmonary veins and their anatomical variations: involvement in video-assisted thoracoscopic surgery-lobectomy for lung cancer. Folia Morphol (Warsz) 2017;76:388-93. [Crossref] [PubMed]
- Fourdrain A, De Dominicis F, Blanchard C, et al. Three-dimensional CT angiography of anatomic variations in the pulmonary arterial tree. Surg Radiol Anat 2018;40:45-53. [Crossref] [PubMed]
- Shiina N, Kaga K, Hida Y, et al. Variations of pulmonary vein drainage critical for lung resection assessed by three-dimensional computed tomography angiography. Thorac Cancer 2018;9:584-8. [Crossref] [PubMed]
- Nagashima T, Shimizu K, Ohtaki Y, et al. An analysis of variations in the bronchovascular pattern of the right upper lobe using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2015;63:354-60. [Crossref] [PubMed]
- Nagashima T, Shimizu K, Ohtaki Y, et al. Analysis of variation in bronchovascular pattern of the right middle and lower lobes of the lung using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2017;65:343-9. [Crossref] [PubMed]
- Deng Y, Cai S, Huang C, et al. Anatomical variation analysis of left upper pulmonary blood vessels and bronchi based on three-dimensional reconstruction of chest CT. Front Oncol 2022;12:1028467. [Crossref] [PubMed]
- Fan K, Feng J, Li Y, et al. Application of three-dimensional reconstruction of left upper lung lobes in anatomical segmental resection. Thorac Cancer 2022;13:1176-83. [Crossref] [PubMed]
- Maki R, Miyajima M, Ogura K, et al. Pulmonary vessels and bronchus anatomy of the left upper lobe. Surg Today 2022;52:550-8. [Crossref] [PubMed]
- He H, Wang F, Wang PY, et al. Anatomical analysis of variations in the bronchus pattern of the left upper lobe using three-dimensional computed tomography angiography and bronchography. Ann Transl Med 2022;10:305. [Crossref] [PubMed]
- Maki R, Miyajima M, Ogura K, et al. Pulmonary vessels and bronchial anatomy of the left lower lobe. Surg Today 2020;50:1081-90. [Crossref] [PubMed]
- Wang LF, Zhao L, Lv CS, et al. Anatomical type analysis of right interlobar artery based on chest thin-slice CT scan and three-dimensional reconstruction. J Cardiothorac Surg 2022;17:328. [Crossref] [PubMed]
- Xu X, Wang J, Liu Q, et al. Clinical significance of the diverse interlobar veins hidden in the upper oblique fissure. Ann Transl Med 2022;10:34. [Crossref] [PubMed]
- Murota M, Yamamoto Y, Satoh K, et al. An analysis of anatomical variations of the left pulmonary artery of the interlobar portion for lung resection by three-dimensional CT pulmonary angiography and thin-section images. Jpn J Radiol 2020;38:1158-68. [Crossref] [PubMed]
- Zhang M, Mao N, Wang SH, et al. The B1 defective type of bifurcated right upper lobe bronchus. J Thorac Dis 2019;11:4218-23. [Crossref] [PubMed]
- Shimizu K, Nagashima T, Ohtaki Y, et al. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen Thorac Cardiovasc Surg 2016;64:604-11. [Crossref] [PubMed]
- Nakazawa S, Shimizu K, Kawatani N, et al. Right upper lobe segmentectomy guided by simplified anatomic models. JTCVS Tech 2020;4:288-97. [Crossref] [PubMed]
- Zhang M, Mao N, Zhang K, et al. Analysis of the variation pattern in left upper division veins and establishment of simplified vein models for anatomical segmentectomy. Ann Transl Med 2020;8:1515. [Crossref] [PubMed]
- Zhong X, Huang Y, Yang M, et al. Elucidating the anatomy of the quadrivial pattern of the right upper lobe bronchus using 3D-CT images. Ann Transl Med 2022;10:46. [Crossref] [PubMed]
- Zhou H, Wei W, He H, et al. A cross-sectional study: analysis of anatomical variation in the right upper lung intersegmental vein V2a based on a 3D reconstruction technique. J Thorac Dis 2022;14:4460-7. [Crossref] [PubMed]
- Miyamoto N, Yoshida M, Takashima M, et al. Classifying the destination of right top pulmonary vein in 31 clinical cases. Gen Thorac Cardiovasc Surg 2021;69:1192-5. [Crossref] [PubMed]
- Miura K, Shimizu K, Mishima S, et al. Anatomical resection for right B(3) downwards-shifting malformation. Gen Thorac Cardiovasc Surg 2023;71:71-5. [Crossref] [PubMed]
- Isaka T, Mitsuboshi S, Maeda H, et al. Anatomical analysis of the left upper lobe of lung on three-dimensional images with focusing the branching pattern of the subsegmental veins. J Cardiothorac Surg 2020;15:273. [Crossref] [PubMed]
- He H, Chen P, Chen X, et al. Analysis of anatomical variations of the lingular artery of the left upper lobe using 3D computed tomography angiography and bronchography. J Thorac Dis 2021;13:5035-41. [Crossref] [PubMed]
- Gao C, Xu WZ, Li ZH, et al. Analysis of bronchial and vascular patterns in left upper lobes to explore the genesis of mediastinal lingular artery and its influence on pulmonary anatomical variation. J Cardiothorac Surg 2021;16:306. [Crossref] [PubMed]
- Liu Y, Zhang S. Mediastinal basal pulmonary artery identification and classification by three-dimensional reconstruction. Surg Radiol Anat 2022;44:447-53. [Crossref] [PubMed]
- Chen X, Wang Z, Qi Q, et al. A fully automated noncontrast CT 3-D reconstruction algorithm enabled accurate anatomical demonstration for lung segmentectomy. Thorac Cancer 2022;13:795-803. [Crossref] [PubMed]
- Hanawa R, Shigenobu T, Tajima A. Three-dimensional reconstruction of pulmonary blood vasculature from plain computed tomography using Ziostation2. Jpn J Chest Surg 2019;33:578-86. [Crossref]
- Nakao M, Omura K, Hashimoto K, et al. Three-dimensional image simulation for lung segmentectomy from unenhanced computed tomography data. Gen Thorac Cardiovasc Surg 2022;70:312-4. [Crossref] [PubMed]
- Nakazawa S, Hanawa R, Nagashima T, et al. Segmentectomy guided by 3-dimensional images reconstructed from nonenhanced computed tomographic data. Ann Thorac Surg 2021;111:e301-4. [Crossref] [PubMed]
- Shimizu K, Nakano T, Kamiyoshihara M, et al. Segmentectomy guided by three-dimensional computed tomography angiography and bronchography. Interact Cardiovasc Thorac Surg 2012;15:194-6. [Crossref] [PubMed]
- Iwano S, Usami N, Yokoi K, et al. Segmentectomy simulation using a virtual three-dimensional safety margin. Ann Thorac Surg 2012;93:e37-9. [Crossref] [PubMed]
- Nakano T, Shimizu K, Nakano S, et al. Usefulness of three-dimensional computed tomographic angiography with bronchography for the planning of minimally invasive video-assisted thoracic surgery for intralobar pulmonary sequestration. Eur J Cardiothorac Surg 2013;43:199. [Crossref] [PubMed]
- Ikeda N, Yoshimura A, Hagiwara M, et al. Three dimensional computed tomography lung modeling is useful in simulation and navigation of lung cancer surgery. Ann Thorac Cardiovasc Surg 2013;19:1-5. [Crossref] [PubMed]
- Saji H, Inoue T, Kato Y, et al. Virtual segmentectomy based on high-quality three-dimensional lung modelling from computed tomography images. Interact Cardiovasc Thorac Surg 2013;17:227-32. [Crossref] [PubMed]
- Iwano S, Yokoi K, Taniguchi T, et al. Planning of segmentectomy using three-dimensional computed tomography angiography with a virtual safety margin: technique and initial experience. Lung Cancer 2013;81:410-5. [Crossref] [PubMed]
- Niu Z, Chen K, Jin R, et al. Three-dimensional computed tomography reconstruction in video-assisted thoracoscopic segmentectomy (DRIVATS): A prospective, multicenter randomized controlled trial. Front Surg 2022;9:941582. [Crossref] [PubMed]
- Sadeghi AH, Maat APWM, Taverne YJHJ, et al. Virtual reality and artificial intelligence for 3-dimensional planning of lung segmentectomies. JTCVS Tech 2021;7:309-21. [Crossref] [PubMed]
- Bakhuis W, Kersten CM, Sadeghi AH, et al. Preoperative visualization of congenital lung abnormalities: hybridizing artificial intelligence and virtual reality. Eur J Cardiothorac Surg 2022;63:ezad014. [Crossref] [PubMed]
Cite this article as: Nakazawa S, Nagashima T, Kawatani N, Gedeon PC, DeSimone AK, Igai H, Kosaka T, Shirabe K. Anatomy of the lung revisited by 3D-CT imaging. Video-assist Thorac Surg 2023;8:17.


