Surgical management of teratoma located in pretracheal retrocaval space: from pre-operative 3D reconstruction to robotic surgery
Introduction
Mediastinal lesions include a wide histopathological variety most frequently located in the anterior compartment (1). Teratoma is one of the most common cancers detected in the mediastinum, characterized by benign nature and slow growth. Mediastinal teratoma is the most common germ cell tumour, frequently located in the anterior mediastinum and diagnosed between 20- and 40-year-old patients (2).
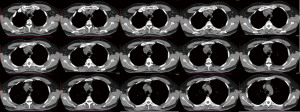
We report a case of mediastinal teratoma located in the pretracheal retrocaval space. The patient is a 47-year-old man referred to the emergency room after an episode of dyspnea in December 2020, in the suspicion of a respiratory severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infection. Chest computed tomography (CT)-scan without contrast agent revealed a mediastinal tumour located in the right superior paratracheal site. The mass appeared as a partially cystic formation of around 4×3 cm in diameter, with regular margins, multiple chambers and internal calcifications (Figure 1).
The patient was a former smoker, with a history of allergic asthma and monoclonal gammopathy of undetermined significance (MGUS).
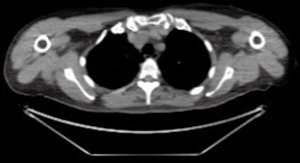
The evaluation of a previous fluorodeoxyglucose-positron emission tomography (FDG-PET) /CT (Figure 2), performed 3 years earlier to investigate potential bone marrow infiltration or unsuspected disease site, demonstrated the presence of the lesion, with the same morphology but smaller, without hypermetabolic activity.
Afterwards, given the ambiguous and heterogenous aspect of the mediastinal mass, endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) was performed, to use firstly the less invasive diagnostic technique: bronchial exploration showed a regular tracheobronchial system, and no atypical cells were found by cytological examination of the samples. Nevertheless, an ultrasound examination performed during the procedure suggested a possible neoplastic nature of the lesion, so the patient was selected for surgical excision.
Surgical techniques
During pre-operative planning, three-dimensional (3D) reconstruction of CT images was obtained, using a free open-source medical image processing software application (3D Slicer; www.slicer.org) supported by a specialized team of engineers, to evaluate the precise localization of the neoplasm and its anatomical relationship with the adjacent vascular structures (Video 1).
A surgical resection of the lesion was decided, after tumour board discussion.
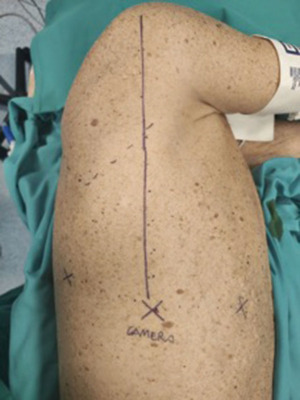
A totally endoscopic 3-ports approach by robotic surgery was planned. A double-lumen endotracheal tube for selective left lung ventilation was positioned to obtain single-lung ventilation. The patient was placed in lateral decubitus, with the operating table tilted upward at the tip of the scapula, in reverse-trendelenburg position of the surgical bed, to maximize the working space and increase the exposure of the surgical field. Port mapping required 3 centimetric incisions, without an assistant port: the camera port was placed at the 7th intercostal space along an imaginary line from the head of the humerus in the intersection with the intercostal space, the second one at the same intercostal space posteriorly to the camera port (about 8 cm), the anterior port at the 6th intercostal space on the anterior axillary line, above diaphragm insertion (Figure 3).
CO2 was inflated using a 5 mmHg pressure to increase the surgical fields and thus the space available for maneuverability. A 30° camera was used and the right upper pretracheal mass, which was approximately 4 cm in diameter, was identified. Macroscopically the lesion was oval regularly shaped with a taut-elastic consistency.
Dissection was performed using two bipolar instruments, a Maryland Bipolar Forceps and a Fenestrated Bipolar Forceps (Intuitive Surgical), introduced via the anterior and posterior ports, respectively.
The mediastinal pleura between the trachea and the superior vena cava was incised and the lesion was isolated by dissection and coagulation maneuvers. Furthermore, EndoWrist Suction/Irrigator (Intuitive Surgical), controlled from the surgeon’s console, was also used to complete the dissection, maintaining a clear vision (Video 2).
The surgical procedure lasted 80 minutes, including the docking time of the robotic system.
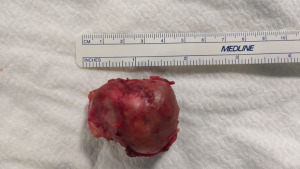
The specimen was removed using an endoscopic bag (Figure 4). At the end of the procedure, a 24 Fr chest tube was placed through the camera port and it was removed on the 1st postoperative day. The patient was discharged on 2nd postoperative day.
The examination of the specimen revealed a pluri-loculated cyst with solid areas. The lesion showed areas of mature pancreatic tissue including endocrine cell islets and exocrine pancreatic parenchyma. Furthermore, a keratinizing squamous epithelium with cutaneous adnexal glands, small intestine, and bronchus including respiratory epithelium, consistent with mature-cystic teratoma, was observed.
The case was subjected to tumour board consultation and the patient was addressed to radiological follow-up.
Ethical statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Tips and tricks
- The reverse-trendelenburg position of the operating table facilitates the downward displacement of the lung parenchyma, providing for an adequate surgical field in terms of vision and movement.
- Gauzes can be useful to retract down the right lung, maintaining a clear and unobstructed vision of the operative area, even in the absence of the fourth robotic arm, usually used as a lung retractor.
- The use of bipolar instruments can reduce vessel injury related to monopolar coagulation. In addition, the bipolar instrument may be used to grasp the tumor for easier manipulation.
- In narrow spaces, small, rolled gauzes can be useful to clean the surgical field and to hold the lesion in place during its mobilization.
- In the proximity of large vessels, such as the superior vena cava, dissection can be also performed with EndoWrist Suction/Irrigator (Intuitive Surgical) manipulated by the surgeon at the console, avoiding the use of electrified instruments and keeping the surgical field dry.
Comments
In the last decade, robotic surgery has been gradually gaining popularity in the thoracic field worldwide (3,4). Specifically, the robotic technique can express the maximum result of its features in the mediastinum, which is an anatomic area characterized by limited surgical space, containing several anatomical structures (5,6). Indeed, the robotic system allows a magnified 3D vision with direct camera control from the surgical console with a great precision of articulation and instruments movements.
Over the years, robotic indication has also been enlarged to more complex cases, in parallel with the increase in surgeons’ experience (7-9). Therefore, preoperative planning of the surgical procedure appears to be crucial to the established operative strategies. In this regard, 3D reconstruction of CT scan images may provide an exhaustive view of anatomical details, which is useful for planning the surgical procedure, reducing the risk of intraoperative unexpected complications.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-22-36/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-22-36/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hazzard C, Kaufman A, Flores R. Mediastinal Tumors. In: Pass HI, Ball D, Scagliotti GV. editors. IASLC Thoracic Oncology. 2nd edition. Elsevier, 2018:550-4.e1.
- Allen MS, Trastek VF, Pairolero PC. Benign germ cell tumors of the mediastinum. In: Shields TW, Locicero J III, Ponn RB. editors. General thoracic surgery. 5th edition. Philadelphia, PA, USA: Lippincott Williams & Wilkins, 2000:2275-82.
- Zirafa CC, Romano G, Key TH, et al. The evolution of robotic thoracic surgery. Ann Cardiothorac Surg 2019;8:210-7. [Crossref] [PubMed]
- Geraci TC, Scheinerman J, Chen D, et al. Beyond the learning curve: a review of complex cases in robotic thoracic surgery. J Thorac Dis 2021;13:6129-40. [Crossref] [PubMed]
- Straughan DM, Fontaine JP, Toloza EM. Robotic-Assisted Videothoracoscopic Mediastinal Surgery. Cancer Control 2015;22:326-30. [Crossref] [PubMed]
- Na KJ, Kang CH. Robotic thymectomy for advanced thymic epithelial tumor: indications and technical aspects. J Thorac Dis 2020;12:63-9. [Crossref] [PubMed]
- Kneuertz PJ, Kamel MK, Stiles BM, et al. Robotic Thymectomy Is Feasible for Large Thymomas: A Propensity-Matched Comparison. Ann Thorac Surg 2017;104:1673-8. [Crossref] [PubMed]
- Chen-Yoshikawa TF, Fukui T, Nakamura S, et al. Current trends in thoracic surgery. Nagoya J Med Sci 2020;82:161-74. [PubMed]
- Gao Y, Jiang J, Xiao D, et al. Robotic-assisted thoracic surgery following neoadjuvant chemoimmunotherapy in patients with stage III non-small cell lung cancer: A real-world prospective cohort study. Front Oncol 2022;12:969545. [Crossref] [PubMed]
Cite this article as: Lenzini A, Zirafa CC, Ceccarelli I, Romano G, Ali G, Capellini K, Davini F, Celi S, Fontanini G, Melfi F. Surgical management of teratoma located in pretracheal retrocaval space: from pre-operative 3D reconstruction to robotic surgery. Video-assist Thorac Surg 2023;8:9.