
Hybrid ablation of atrial fibrillation in patients with prior coronary artery bypass grafting: a single center retrospective cohort study
Highlight box
Key findings
• A hybrid ablation approach for AF in patients with prior CABG is technically possible and associated with a high rhythm success rate.
• HRS success (<30 s AF/AT/AFl off AAD): 82.3%.
What is known and what is new?
• A hybrid approach is associated with better rhythm success outcomes than isolated endocardial catheter ablation in patients with non-paroxysmal AF.
• In patients with prior CABG a hybrid approach is unreported.
What is the implication, and what should change now?
• Prior CABG may not be a strict contra-indication to a hybrid approach.
• Patients with prior surgery should be considered for a hybrid approach.
Introduction
Background
Atrial fibrillation (AF) remains the most common cardiac arrhythmia, with a rising prevalence as the global population continues to age (1). Cardiothoracic surgeons are poised to assume a more significant role in the care of patients with AF as recent randomized control studies, including the CONVERGE trial (2), show the superiority of a hybrid (epicardial and endocardial ablation) team (cardiothoracic surgeon and electrophysiologist) approach over an isolated endocardial approach in patients with non-paroxysmal (persistent and long standing persistent) AF.
Moreover, new-onset post-operative AF (POAF) is a common complication after cardiac surgery, with prevalence ranging from 30–50% (3). The most common method to achieve rhythm control of POAF is pharmacologic intervention with amiodarone (4), while electrocardioversion is also utilized in approximately 25% patients who do not respond to medical intervention (5). The possible sequala of embolic phenomena [cerebral vascular accident (CVA)/transient ischemic attack (TIA)] or systemic emboli from POAF is most commonly treated with guideline directed oral anticoagulation (OAC) (6). With these interventions, in most patients POAF resolves with routine care during the immediate 30-day post-operative period (4), however in some patients POAF persists and may require further intervention (7,8). Factors associated with sustained POAF despite pharmacological intervention or electrocardioversion include increased age, prior myocardial infarction, a history of congestive heart failure and elevated EuroSCORE II (5).
In addition, unfortunately a majority of patients that enter the cardiac operating room with a concomitant diagnosis of AF will not undergo a concomitant surgical ablation (9-12) and according to the most recent Society of Thoracic Surgeons (STS) database review, nearly 40% leave the cardiac operating room without management of the left atrial appendage (LAA) (13). Specifically, patients undergoing coronary artery bypass grafting (CABG) surgery have abysmal rates [16.2% (11) and 16.4% (9)] of concomitant surgical ablation. Reasons attributed to the omission of surgical ablation for AF at the time of concomitant cardiac surgery include but are not limited to; a perceived lack of benefit of surgical ablation {despite STS and the American Association for Thoracic Surgery (AATS) class IA mitral valve replacement (MVR) and IB [CABG-aortic valve replacement (AVR)] guideline recommendations}, the need for additional cross-clamp time, perceived procedural risk from additional “unnecessary” atriotomies (left and right), and perceived patient “high” risk factors such as increased age, renal insufficiency, depressed ejection fraction, and overall increased STS predicted risk of mortality (PROM) score (9,14-17). At the time of concomitant surgical ablation for pre-existing AF; the most common surgical ablation lesions are fundamentally based on the principles of the Cox-Maze 3 or 4 operations and include bilateral pulmonary vein isolation and interconnecting lesions between the superior pulmonary veins and inferior pulmonary veins respectively, to isolate the posterior left atrium and exclusion of the LAA. Unfortunately, less often performed lesions also include; overlapping mitral isthmus and coronary sinus ablations, ablation of the “coumadin ridge” or the tissue ridge between the base of the LAA and the left superior pulmonary vein, the tricuspid valve isthmus, an intercaval ablation between the superior vena cava (SVC) and inferior vena cava (IVC), and a “T”-lesion connecting the right atrial appendage to the free wall of the right atrium and the intercaval ablation. The LAA is most commonly excluded via cut and sew, stapler exclusion, suture ligature or epicardial clip device placement (18). This leaves thousands of patients that may require either endocardial or redo-cardiac intervention to achieve rhythm control and LAA management.
Rationale and knowledge gap
Limited data exist regarding the safety and effectiveness of catheter ablation in the setting of prior-cardiac surgery (19) and to our knowledge no literature exists regarding the role of hybrid ablation and LAA management in the prior-CABG surgery population. Hybrid ablation is an emerging technique utilized in patients with complex AF who have often not responded to typical medical management or isolated endocardial ablation of the pulmonary veins. As aforementioned, in a randomized control study (CONVERGE), a hybrid ablation approach has been demonstrated to be superior to isolated endocardial ablation in patients with non-paroxysmal AF (persistent and long-standing persistent). The combined epicardial and endocardial approach appears to confer an improved ability to achieve ablation transmurality and subsequent tissue isolation with reduction in AF burden and restoration of normal sinus rhythm.
Objective
We have previously demonstrated the safety and efficacy of our hybrid ablation approach on the treatment of AF (20). In this study, we seek to determine if in the hands of an experienced hybrid team, if the hybrid ablation approach is safe and effective for those with a history of prior CABG. We present the following article in accordance with the STROBE reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-22-34/rc).
Methods
Data collection
Patient demographics and standard post-surgical outcomes (death, stroke, infections, etc.) are collected via an Adventist Health independent 3rd party data abstraction team and inserted into our STS database. AF specific outcomes [AF, atrial tachycardia (AT), atrial flutter (AFl), anti-arrhythmia drugs (AADs) use, anti-coagulation use, etc.] are collected via our data team (co-authors: CP, MD, AP) and inserted into our password protected secure institutional AF database. AF outcomes and STS data are combined after institutional review board approval to provide complete patient data. Data were collected retrospectively via chart review. Patient data were reviewed for study eligibility from 2013 to 2018 at Adventist Health-Saint Helena Hospital, Saint Helena, Napa Valley, CA, USA. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Adventist Health-Saint Helena Institutional Review Board on 1/21/2020, approved this study and individual consent for this retrospective analysis was waived.
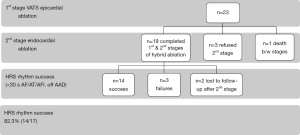
All adult patients (>18 years of age) in our AF database between 2013 to 2018 who underwent the 1st stage epicardial video-assisted thoracoscopic surgery (VATS) surgical ablation for isolated AF were reviewed for eligibility (n=455). Patients who then had a prior isolated CABG were then specifically reviewed to be included in this study (n=23). Patients who completed both stages of the hybrid approach (epicardial VATS and endocardial ablation) (Figure 1) and at least 24 h of continuous ambulatory rhythm monitoring were then reviewed for rhythm success [Heart Rhythm Society (HRS) <30 s of AF, AT, AFl and off AAD with 24-h of continuous ambulatory monitoring] (n=17). Patients who failed to undergo the 2nd stage of the intended combined hybrid approach were excluded from the rhythm success category (Figure 2).
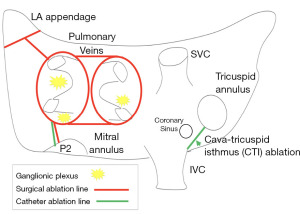
Rhythm monitoring was performed by the Saint Helena Hospital Arrhythmia Center team at regular intervals of 3- and 12-month post-operatively and annually thereafter and monitored by the AF nurse navigator (co-author: CP). Rhythm data were obtained from standard permanent pacemaker (PPM) interrogations and transdermal Ziopatch monitors, which provide continuous 24-h rhythm data for up to 14-day. Rhythm data were then reviewed by our research team and adjudicated as a “rhythm success” if it met HRS criteria of less than 30 s of AF, AT, or AFl without the use of class I or III AAD. iRhythm Zio patch monitoring is an industry standard transdermal electrogram monitoring device that provides 24-h of continuous electrogram monitoring for up to 14-day. iRhythm provides patient rhythm monitor reports at the end of patch use to the prescribing physicians (co-authors: AK, SE, GD). Pacemaker interrogations are standard practice protocols to evaluate the arrhythmia burden capture by patient PPMs. Rhythm data are inserted into the electronic medical record and adjudicated by the Saint Helena Arrhythmia Team (6). One patient refused >24 h monitoring so only electrocardiogram (ECG) data was available and this patient is defined as an HRS failure despite normal sinus rhythm on ECG. Hybrid ablation was performed as our group has previously reported (20) and depicted in Figure 1.
Statical analysis
Statistical analysis and creation of figures were performed using GraphPad Prism 9.2.0 [283], GraphPad Software, San Diego, CA, USA. Total study cohort, n=23. All results are described as mean ± standard deviation (SD) unless otherwise noted.
Adventist Health-Saint Helena Hospital is located in Napa Valley, CA, USA. A significant portion of our patient referrals travel >50 miles (80 km) to our facility for treatment, with 53% traveling at least 3 h by automobile. Patient follow-up is challenging. Patients were seen in the outpatient setting at 2 weeks, 3 months and annually thereafter. Two patients were lost to follow-up after completion of the 2nd stage.
Results
Patient demographics
Complete patient demographics are described in Table 1. Most patients were male gender 21/23 (91%) and all suffered from non-paroxysmal AF (48% persistent, 52% long-standing persistent). The average time in AF prior to hybrid ablation was 3.2±3.4 years. The average pre-hybrid ablation left ventricular ejection fraction (LVEF)% was 46.3±10.2 and left atrial size was 5.1±0.8 cm. Most patients at the time of surgery were maintained on OAC therapy prior to hybrid ablation (20/23, 87%) and 30% were on class I or III AAD, the remaining 70% had been transitioned to rate control agents after failing prior class I or III AAD by their referring physician.
Table 1
| Patient attributes | Value |
|---|---|
| Age (years) | 73.8±10.2 [40–87] |
| BMI (kg/m2) | 30.2±5.55 [20.3–40.6] |
| Gender (male: female) | 21:2 |
| LVEF (%) | 46.3±10.2 |
| LA size (cm) | 5.1±0.8 |
| AF | |
| Persistent | 11 [48] |
| Long standing persistent | 12 [52] |
| Time in AF (years) | 3.2±3.4 |
| Pre-procedural | |
| OAC | 20 [87] |
| Class I or III AAD | 7 [30] |
| Pacemaker | 4 [17] |
| CHA2D-VaS2C score | 4.4±1.5 |
| HASBLED score | 3.0±0.8 |
Data are presented as mean ± SD [range], n, mean ± SD, or n [%]. BMI, body mass index; LVEF, left ventricular ejection fraction; LA, left atrial; AF, atrial fibrillation; OAC, oral anticoagulation; AAD, anti-arrhythmia drug; SD, standard deviation.
Procedural outcomes
Procedural outcomes are outlined in detail in Table 2. The 1st stage VATS epicardial hybrid ablation required on average 2.8±0.5 hours to complete. At the 1st stage all patients underwent VATS bilateral pulmonary vein isolation and left atrial posterior wall isolation with interconnecting lesions (roof and floor). Nineteen patients completed the 2nd stage of the hybrid approach. At the time of the 2nd stage endocardial mapping, but prior to further endocardial ablation, the 1st stage epicardial ablation resulted in 73.6% (14/19) endocardial isolation of bilateral pulmonary veins and 47.4% (9/19) endocardial isolation of the left atrial posterior wall or “box”. The right inferior pulmonary vein (incomplete 3/19, 15.7%) was the most resistant to transmural isolation at the 1st stage in this cohort. Creation of transmural and continuous roof (incomplete 7/19, 37%) and floor lesions (incomplete 6/19, 32%) was also challenging in this cohort.
Table 2
| Procedural parameters | Value |
|---|---|
| 1st VATS stage—surgical time (hours) (n=23)† | 2.8±0.5 |
| Length of stay (days) (n=23)† | 4.2±2.3 |
| 2nd stage mapping prior to endocardial ablation (n=19)† | |
| Pulmonary vein isolation complete after 1st stage | 14/19 (73.6) |
| Left atrial “box” complete after 1st stage | 9/19 (47.4) |
| VATS LAA clip (n=22)† | |
| 35 mm Pro 2 | 15/22 (68.2) |
| 40 mm Pro 2 | 2/22 (9.1) |
| 40 mm Pro V | 1/22 (4.5) |
| 45 mm Pro 2 | 1/22 (4.5) |
| 45 mm Pro V | 3/22 (13.6) |
| New pacemaker (n=23)† | 3/23 (13.0) |
| Off class I or III anti-arrhythmic at last follow-up (n=21)† | 20/21 (95.2) |
| Complications (n=23)† | |
| Death | 1/23 (4.3) |
| Conversion to mini-thoracotomy | 1/23 (4.3) |
| Rhythm success (n=17)† | |
| follow-up (days) | 517 [162–1,538] |
| Success (HRS <30 s AF/AT/AFl, off AAD) | 14/17 (82.4) |
†, n=23 total; n=22, 1 prior LAA-clip; n=19, completed both stages of hybrid approach; n=21, rhythm follow-up; n=17, 2 lost to follow-up after the 2nd stage. Data are presented as mean ± SD, n/N (%), mean [range]. VATS, video-assisted thoracoscopic surgery; LAA, left atrial appendage; HRS, Heart Rhythm Society; AF, atrial fibrillation; AT, atrial tachycardia; AFl, atrial flutter; AAD, anti-arrhythmia drug; SD, standard deviation.
LAA management
LAA was managed previously in one patient. The remaining 22 patients received a VATS AtriClip placement to exclude the LAA. Most patients (18/22, 82%) received an AtriClip Pro 2 and 4 patients received an AtriClip Pro V due to dense adhesion at the tip of the LAA (Table 2). The Pro V clip is an open ended epicardial clip that can be inserted at the base of the LAA without complete mobilization of the appendage and can be advantageous in those patients with dense adhesions at the tip of the appendage near the left pulmonary artery. LAA complete exclusion was evaluated by intra-operative transesophageal echocardiography (TEE) and successful in all patients as defined by <1 cm residual pouch, no device related leak, and no evidence of device related thrombus.
Procedural complications
One patient who had prior LAA ligation with an epicardial cerclage suture tie had particularly dense adhesions at the base of the appendage and developed LAA bleeding during appendage manipulation and clip placement. This patient required conversion to a mini-thoracotomy in order to gain hemostatic control and ultimately accomplish LAA ligation. Three patients developed symptomatic bradycardia post-hybrid ablation and required PPM placement.
One patient death occurred in this cohort. A 74-year-old male who had CABG 10 years prior and had undergone two prior endocardial catheter ablations without success and was on chronic amiodarone therapy for years with subsequent pulmonary toxicity requiring home oxygen therapy. He also had a history of lymphoma status post chemo-radiation therapy. At the time of the 1st stage epicardial ablation he had dense pulmonary adhesions and significant adhesion lysis was required to safely gain access to the pericardium. Ultimately, the ablation procedure was completed, however post-operatively he struggled after his initial extubation and required reintubation on post-operative day #3. We feel the combination of his underlying pulmonary disease and extensive lysis of adhesions may have led to his deteriorated post-operative pulmonary condition. He went on to develop an acute abdomen requiring exploratory laparotomy and despite maximal efforts, on post-operative day #7 the patient expired from multi-system organ failure.
Rhythm success
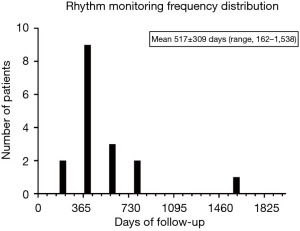
Rhythm success was defined at last follow-up [average 517±309 (range, 162–1,538) days, Figure 3] and defined by standard HRS guidelines of <30 s of AF/AT/AFl and off class I or III AAD. Rhythm monitoring (total cohort n=23, less n=2 lost to follow-up) was evaluated either by PPM interrogation (6 total; 3 prior PPM and 3 new PPM) or >24 h Ziopatch (13 Ziopatch monitors). Two patients were lost to follow-up after the 2nd stage endocardial ablation. Seventeen total patients were evaluated for rhythm success. Fourteen of seventeen patients demonstrated HRS rhythm success (82.3%).
At last follow-up most patients were off class I or III AAD (20/21, 95.2%).
Discussion
Key findings
Approximately 5% (23/455) of patients planned for hybrid ablation at our center had prior CABG. Nineteen of these patients completed both stages of the hybrid ablation approach. Two patients were lost to follow-up after their 2nd stage. Fourteen of seventeen patients (82.3%) demonstrated HRS success at last follow-up. One patient death occurred and one conversion to mini-thoracotomy were required. No strokes occurred. The LAA was successfully occluded in all patients.
Strengths and limitations
Our study has several limitations. We have a small study cohort of 23 total patients. This severely limits are ability to model predictors of rhythm success and to claim generalizability of our findings. The study by its nature is also retrospective and is fraught with confounders and bias that we are not able to control for in our analysis. We were not able to control for surgeon selection bias, patient referral bias from our referring electrophysiologist, prior endocardial ablation techniques, patient preference for continuation to the 2nd stage, and patient compliance with monitoring requests. Finally, our study is limited in its follow-up. Although we go to great lengths to try to ensure perfect follow-up, we provide care in a rural setting in Northern California and this creates challenges for follow-up.
Comparison with similar researches
No prior studies have reported on the utilization of a hybrid approach in the prior CABG population. However, it is important to acknowledge that the Cox-Maze IV surgical ablation for AF is the gold standard and most effective on-pump approach for treating AF. Unfortunately, large Cox-Maze IV series that have examined “high-risk” populations have omitted the redo-population (22) and make comparisons to on-pump Cox-Maze IV results challenging. However, a similar sized on-pump arrested Cox-Maze IV cohort study, n=42, by Kobayashi et al. showed rhythm success results with 67% success at a mean follow-up of 25.5±10.8 months (23).
In June 2021, Dr. Whitlock et al. published the landmark LAAOS III (LAA occlusion study) in the N Engl J Med (18). This was the first randomized control study that demonstrated the effective stroke reduction (hazard ratio, 0.67; 95% confidence interval, 0.53 to 0.85; P=0.001) that occurs with management of the LAA during cardiac surgery in patients with pre-operative AF. Unfortunately, prior to the LAAOS III several studies have reported the lack of LAA management in this same population (10,13). Hopefully, LAAOS III will heighten the collective awareness of the importance of managing the LAA during cardiac surgery, but it is unlikely that adherence to this practice recommendation will be 100%. This will leave a cohort of patients with a LAA that may contribute to further AF related cerebral embolic events. In our redo-population, we have demonstrated a 100% successful ligation of the LAA using the AtriClip. It goes without saying that the LAA is a friable structure and great care needs to be taken to approach the appendage and its mobilization in the redo-setting, however we have shown that it can be done safely and effectively (Video 1). The key to LAA mobilization in the redo-setting is to carefully dissect the appendage with soft, blunt instruments like an endo-Kittner. Significant time (~20 min) may be required to adequately mobilize the appendage in order to place the AtriClip at the true base but the added benefit to the procedure and the patient in exchange for additional operative in time in our estimation is well worth it.
Explanation of findings
The epicardial surgical ablation component of the hybrid approach requires a high-degree of comfort with thoracoscopic surgery and minimally invasive techniques in general. The rate of conversion from VATS to open surgery is often utilized as a marker for procedural safety. In this patient series of 23 patients that had undergone prior CABG via sternotomy we experienced zero conversions to sternotomy and only one conversion to left mini-thoracotomy to address LAA bleeding in a patient who had prior attempted LAA exclusion via an epicardial cerclage suture. We have learned that the AtriClip Pro V can be a useful tool for appendage exclusion in this prior sternotomy population. The LAA can often be adhered to the left pulmonary artery or bypass grafts [especially vein graft to an obtuse marginal target or even a lateral swaying left internal mammary artery (LIMA) to left anterior descending (LAD)] in prior sternotomy patients. The mobilization of the entire appendage in these scenarios can be quiet challenging and the ability to develop a plane-tunnel at the base of the appendage and avoid dissection near the most distal extent (tip) of the appendage can be a useful maneuver to avoid complications. The Pro V clip can then be positioned through that plain-tunnel and exclude the appendage with standard TEE guidance. Also, in terms of bleeding from the LAA, as this is a low-pressure system applying pressure via a 4×4 gauze can often mitigate bleeding from the friable appendage tissue and allow for continued mobilization and successful AtriClip without conversion to mini-thoracotomy or cardiopulmonary bypass.
As aforementioned, one death occurred in this patient cohort. In retrospect, we have learned that the patient with severe lung disease requiring home oxygen is at prohibitive risk for our hybrid approach. During the epicardial VATS surgical ablation, sequential single lung ventilation is required to appropriately access each pericardial “hemisphere” in order to complete bilateral pulmonary vein isolation and creation of the left atrial box. It appears that those with pulmonary insufficiency are primed to poorly respond and are likely to develop post-operative respiratory complications as our patient did. Moving forward, we no longer offer this approach to patients who require home oxygen and we perform pulmonary function tests on all patients preoperatively.
The rhythm success of the hybrid approach in the prior CABG patient was 83.2% (14/17). Interestingly, at the time of the 2nd stage mapping, 74% of bilateral pulmonary veins were isolated after the 1st epicardial ablation only and only 47% of the left atrial “box” was isolated after the first stage epicardial approach. It appears that the prior CABG confers increased resistance to the epicardial ablation when compared to our previously reported “virgin” hybrid ablation series of 86% and 65%, respectively (20). The likely mechanism of this resistance is the presence of epicardial adhesions that interrupt energy delivery. Fortunately, however, 2nd stage endocardial ablation was able to complete transmurality of these lesions and ultimately confer isolation of all pulmonary veins and posterior walls. Moreover, this led to 95% (20/21) freedom from class I or III anti-arrhythmic at last follow-up.
Implications and actions needed
Off-pump AF surgeries, such as the hybrid ablation approach continue to gain popularity as is evident in the most recent STS data review of stand-alone AF surgeries in North America (24). Patients undergoing cardiac surgery unfortunately still are often undertreated in the setting of pre-existing AF. It appears that a hybrid ablation approach may provide a unique solution to the treatment of persistent AF in patients with prior heart surgery, specifically CABG. Moving forward, careful patient selection with a focus on patients with preserved respiratory function may allow for the successful treatment of these patients.
Conclusions
Prior CABG surgery with sternotomy is not a prohibitive barrier to rhythm control success with a hybrid ablation approach strategy. Successful LAA management with freedom from anti-arrhythmic in this population is achievable.
Acknowledgments
Funding: This work was supported by an institutional (Adventist Health-Saint Helena) research grant from AtriCure which provided salary support for our hospital employed research assistants (SB, MD, AP).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-22-34/rc
Data Sharing Statement: Available at https://vats.amegroups.com/article/view/10.21037/vats-22-34/dss
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-22-34/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-22-34/coif). All authors acknowledge that this work was supported by an institutional (Adventist Health-Saint Helena) research grant from AtriCure which provided salary support for their hospital employed research assistants (SB, MD, AP). AK and GHD also provide consultation, teaching and proctoring of hybrid procedures for AtriCure. SE provides consultation and teaching of hybrid procedures for AtriCure. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Adventist Health-Saint Helena Institutional Review Board on 1/21/2020, approved this study and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morillo CA, Banerjee A, Perel P, et al. Atrial fibrillation: the current epidemic. J Geriatr Cardiol 2017;14:195-203. [PubMed]
- DeLurgio DB, Gill JS, Ahsan S, et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-standing Persistent Atrial Fibrillation. Arrhythm Electrophysiol Rev 2021;10:198-204. [Crossref] [PubMed]
- Echahidi N, Pibarot P, O'Hara G, et al. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol 2008;51:793-801. [Crossref] [PubMed]
- Gillinov AM, Bagiella E, Moskowitz AJ, et al. Rate Control versus Rhythm Control for Atrial Fibrillation after Cardiac Surgery. N Engl J Med 2016;374:1911-21. [Crossref] [PubMed]
- Rezk M, Taha A, Nielsen SJ, et al. Clinical Course of Postoperative Atrial Fibrillation After Cardiac Surgery and Long-term Outcome. Ann Thorac Surg 2022;114:2209-15. [Crossref] [PubMed]
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125-51. [Crossref] [PubMed]
- Björn R, Nissinen M, Lehto J, et al. Late incidence and recurrence of new-onset atrial fibrillation after isolated surgical aortic valve replacement. J Thorac Cardiovasc Surg 2022;164:1833-43.e4. [Crossref] [PubMed]
- Abdelmoneim SS, Rosenberg E, Meykler M, et al. The Incidence and Natural Progression of New-Onset Postoperative Atrial Fibrillation. JACC Clin Electrophysiol 2021;7:1134-44. [Crossref] [PubMed]
- McCarthy PM, Davidson CJ, Kruse J, et al. Prevalence of atrial fibrillation before cardiac surgery and factors associated with concomitant ablation. J Thorac Cardiovasc Surg 2020;159:2245-53.e15. [Crossref] [PubMed]
- Badhwar V, Rankin JS, Ad N, et al. Surgical Ablation of Atrial Fibrillation in the United States: Trends and Propensity Matched Outcomes. Ann Thorac Surg 2017;104:493-500. [Crossref] [PubMed]
- Iribarne A, DiScipio AW, McCullough JN, et al. Surgical Atrial Fibrillation Ablation Improves Long-Term Survival: A Multicenter Analysis. Ann Thorac Surg 2019;107:135-42. [Crossref] [PubMed]
- Mehaffey JH, Krebs E, Hawkins RB, et al. Variability and Utilization of Concomitant Atrial Fibrillation Ablation During Mitral Valve Surgery. Ann Thorac Surg 2021;111:29-34. [Crossref] [PubMed]
- Friedman DJ, Piccini JP, Wang T, et al. Association Between Left Atrial Appendage Occlusion and Readmission for Thromboembolism Among Patients With Atrial Fibrillation Undergoing Concomitant Cardiac Surgery. JAMA 2018;319:365-74. [Crossref] [PubMed]
- Badhwar V, Rankin JS, Damiano RJ Jr, et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann Thorac Surg 2017;103:329-41. [Crossref] [PubMed]
- Ad N, Damiano RJ Jr, Badhwar V, et al. Expert consensus guidelines: Examining surgical ablation for atrial fibrillation. J Thorac Cardiovasc Surg 2017;153:1330-54.e1. [Crossref] [PubMed]
- Mehaffey JH, Charles EJ, Berens M, et al. Barriers to atrial fibrillation ablation during mitral valve surgery. J Thorac Cardiovasc Surg 2023;165:650-8.e1. [Crossref] [PubMed]
- Malaisrie SC, McCarthy PM, Kruse J, et al. Ablation of atrial fibrillation during coronary artery bypass grafting: Late outcomes in a Medicare population. J Thorac Cardiovasc Surg 2021;161:1251-61.e1. [Crossref] [PubMed]
- Whitlock RP, Belley-Cote EP, Paparella D, et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N Engl J Med 2021;384:2081-91. [Crossref] [PubMed]
- Nabar A, Timmermans C, Medeiros A, et al. Radiofrequency ablation of atrial arrhythmias after previous open-heart surgery. Europace 2005;7:40-9. [Crossref] [PubMed]
- Dunnington GH, Pierce CL, Eisenberg S, et al. A heart-team hybrid approach for atrial fibrillation: a single-centre long-term clinical outcome cohort study. Eur J Cardiothorac Surg 2021;60:1343-50. [Crossref] [PubMed]
- Kiankhooy A, Pierce C, Burk S, et al. Hybrid ablation of persistent and long-standing persistent atrial fibrillation with depressed ejection fraction: A single-center observational study. JTCVS Open 2022;12:137-46. [Crossref] [PubMed]
- Ad N, Holmes SD, Pritchard G, et al. Association of operative risk with the outcome of concomitant Cox Maze procedure: a comparison of results across risk groups. J Thorac Cardiovasc Surg 2014;148:3027-33. [Crossref] [PubMed]
- Kobayashi J, Kosakai Y, Isobe F, et al. Rationale of the Cox maze procedure for atrial fibrillation during redo mitral valve operations. J Thorac Cardiovasc Surg 1996;112:1216-21; discussion 1222. [Crossref] [PubMed]
- Ad N, Holmes SD, Roberts HG Jr, et al. Surgical Treatment for Stand-Alone Atrial Fibrillation in North America. Ann Thorac Surg 2020;109:745-52. [Crossref] [PubMed]
Cite this article as: Kiankhooy A, Pierce CL, Eisenberg S, Burk S, Daw M, Phillips A, Dunnington GH. Hybrid ablation of atrial fibrillation in patients with prior coronary artery bypass grafting: a single center retrospective cohort study. Video-assist Thorac Surg 2023;8:2.


