
Postoperative delirium in older adults: a surgeon’s guide and clinical practice review
Introduction
Postoperative delirium (POD) is acute dysfunction of cognition and attention that occurs in older patients following major surgery. POD is associated with prolonged hospitalization, increased risk of readmission and nursing home placement, and impaired physical functioning causing about $185 billion per year in healthcare costs in the United States (1-4). As the population ages and more older adults undergo major surgeries (5), POD is increasingly burdening health systems (6,7). Thus, it is imperative that surgeons understand how to prevent, recognize, and treat POD. The purpose of this review is to provide a concise synopsis of key concepts and present simple practice modifications that surgeons can implement when caring for their older patients. The content of this piece has been selected and assembled by experts in the fields of surgery, geriatrics, and geriatric surgery—it is not the product of a systematic review.
Incidence and risk factors
The incidence of POD varies widely based on the patient population, ranging up to 77% (8-11). POD occurs more frequently after orthopedic (12), cardiac (9,11), and noncardiac thoracic surgeries (9).
There are many known risk factors for POD at each stage of operative care (Table 1), a selection of which are discussed in this review. Age is one of the most prominent risk factors, with patients >70 years old at increased risk of POD (13). Preoperative risk factors also include malnutrition, preexisting cognitive impairment, impaired functional status, substance abuse, and anemia (7,8,14-16). Prolonged operative time (8,17), required blood transfusion (18), and type of surgery are intraoperative risk factors for POD. Postoperatively, both uncontrolled pain (19,20) and high doses of opioids (20) increase the incidence of POD, in addition to immobilization (21), electrolyte derangements (22), infections (2), anticholinergic medications (23), and benzodiazepines (24).
Table 1
| Predisposing factors |
| Age >70 years |
| Malnutrition |
| Limited functional status |
| Baseline cognitive dysfunction |
| Sensory impairment |
| Substance abuse |
| Depression |
| Anemia |
| Diabetes |
| Peripheral vascular disease |
| Precipitating factors |
| Antihistamine |
| Tricyclic antidepressant |
| Benzodiazepine |
| Analgesic |
| Bronchodilator |
| Bladder control |
| Antiparkinsonian |
| Infection |
| Dehydration |
| Trauma |
| Surgery |
| Emotional stress |
| Sleep deprivation |
| Hydroxyzine, diphenhydramine |
| Amitriptyline |
| Lorazepam |
| Codeine |
| Theophylline |
| Oxybutynin |
| Benztropine |
| Intraoperative factors |
| Urgency of procedure |
| Duration of procedure |
| Hypotension |
| Blood loss |
| Blood transfusion |
| Electrolyte imbalance |
| Blood transfusion requirement |
| Hypoxemia |
| Pain |
| Lack of mobilization |
| Sleep/wake disturbances |
| Missing hearing aids or other devices required for communication |
Presentation and diagnosis
POD can present immediately after anesthesia in the post-anesthesia care unit through hospital discharge (25). Cases of POD vary in length and etiology. Previous studies indicate that >50% of POD cases go undocumented; false-negative rate is >80% on the day of surgery and the first day after surgery (26).
Recognized cases of POD often present as a change from a patient’s baseline mental status, characterized by disorientation and fluctuations in attention. POD can be classified as one of three subtypes: hyperactive, characterized by agitation; hypoactive, characterized by lethargy; or mixed, fluctuating between hyperactivity and hypoactivity (27,28). Hypoactive delirium is most common in older patients, followed by mixed presentation, while pure hyperactive delirium is rare (29). It is important to distinguish hypoactivity, lethargy, and sluggishness associated with delirium from symptoms related to the effects of anesthesia. Other symptoms of POD can include difficulty focusing, hallucinations, slurred speech, rapid mood swings, and restlessness (30).
The first step in assessing a patient who may be experiencing POD is to determine their level of arousal, often done using the Richmond Agitation Sedation Scale (RASS) (31). If the patient is unarousable (RASS score: −5) or in a state of deep sedation (RASS score: −4), they should be immediately assessed for ability to protect their airway and for transfer to a higher level of care. Once stabilized, a broad workup for unresponsiveness should be performed, as isolated POD itself does not cause life-threatening changes in mental status.
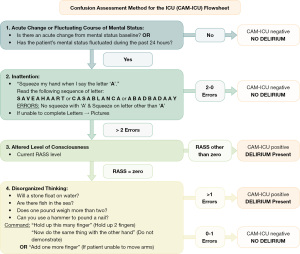
For arousable patients with RASS scores of −3 or higher, assessment for delirium is the next step. The 3-Minute Diagnostic Confusion Assessment Method (3D-CAM) (32-34) and Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) (35) are most commonly used. These tools give a stepwise approach to assess attention, level of consciousness, and disorganized thinking (Figure 1). Based on a patient’s ability to perform certain tasks, they are considered delirious (CAM positive) or not delirious (CAM negative). The 3D-CAM model is used for patients who can verbally respond to questions while the CAM-ICU sequence can be used for patients who are intubated or unable to speak. These assessments are optimally performed and documented by nurses on a daily basis (35).
Prevention
Prevention is the best treatment for POD. Patients should be frequently re-oriented to time and place, the reason for their hospitalization, and the treatment they are receiving. Pain levels should be closely monitored, as uncontrolled pain is a common cause of POD (36,37). However, excess dosing of narcotic pain medications can also precipitate or exacerbate delirium (9); accordingly, it is important to use a multimodal pain regimen to spare opioid use and assess patients both before and after administration of narcotics and to adjust opioid dosing accordingly. Patients’ intake and output should be tracked carefully to identify, prevent, and/or treat constipation and urinary retention (9,37). In many patients, a prophylactic bowel regimen is helpful.
Sensory aids (e.g., hearing aids, glasses, dentures) should be returned to patients immediately after surgery to facilitate communication and acclimation to their environment (9,37). Patients also should be mobilized as early as possible after surgery (38). The sleep/wake cycle should be maintained as best as possible in the hospital environment by limiting alarms, connections to lines and monitors, and collection of vital signs overnight to interventions that are absolutely necessary. In addition, lights and televisions should be turned off during the night, while window shades should be opened during the day (9,37). Engaging the patient’s family in these preventive strategies is often helpful and having family or friends present at bedside can assist with orientation (38). Music therapy and pet therapy have also been shown to improve mood and patient engagement. (39,40).
Frequent and focused review of medications can limit exposure to medications that might contribute to POD. In general, it is best to avoid anticholinergics (i.e., amitriptyline, oxybutynin, scopolamine), antihistamines (i.e., diphenhydramine, hydroxyzine), and benzodiazepines (i.e., diazepam, lorazepam, alprazolam), especially in older patients (41). In addition, it is important to resume certain home medications as soon as possible to avoid withdrawal, including in those patients with underlying dementia treated with cholinesterase inhibitors (e.g., donepezil, rivastigmine) as well as those on dopamine agonists (e.g., carbidopa-levodopa) for Parkinsonism (9,42).
Preventative measures are often best implemented with multidisciplinary care. Many care models including geriatric surgery co-management models between geriatrics and surgery teams have been successfully tested and deployed in the postoperative setting (43-45). The Geriatric Surgery Verification Program (GSVP), born out of the partnership between the American College of Surgeons and the John A. Hartford Foundation, is one such model. GSVP specifies 32 standards to optimize preoperative, intraoperative, and postoperative care of older adults (46). Early data show that patients cared for with the GSVP multidisciplinary model experience lower rates of POD (47).
Management
Perhaps the most important component of managing POD is to treat the underlying causes (9,36) optimally via comprehensive evaluation including a current history, physical examination with emphasis on neurologic assessment, appropriate laboratory testing and imaging, and review of current medications. The basic medical assessment should include a complete blood count to evaluate for anemia and infection, a metabolic panel to assess renal function, and urinalysis (9). Patients also should be evaluated for uncontrolled pain, constipation, and urinary retention (9,37). For patients without focal neurologic symptoms, computed tomography of the head is considered to be of low diagnostic value to assess POD (9). Specific consideration should be given to the potential need for reversal of anesthetic agents that may have prolonged effects.
Once potential underlying causes have been addressed, nonpharmacologic interventions should be pursued. The mainstays of POD treatment are the same elements discussed above as preventative measures, including frequent re-orientation, early return of sensory aids, early mobilization, and normalization of sleep/wake cycles.
Pharmacotherapy is reserved for agitated patients who are a physical threat to themselves or others despite behavioral interventions and nonpharmacologic treatments. These drugs do not treat or reverse POD but rather sedate patients to help control unsafe behaviors (48). When pharmacologic treatment is necessary, it is important to evaluate for continued need on a daily basis (36).
Antipsychotics are the pharmacologic treatment of choice when required for POD. Haloperidol, a typical antipsychotic, is used by many as a first-line agent (9,37). Haloperidol can reduce the length of episodes of agitated delirium (49). It is recommended to start with a low dose (0.5–1 mg) and repeat dosing as necessary (9,37,50). Atypical antipsychotics such as risperidone, olanzapine, and quetiapine are alternatives to haloperidol and are preferred in certain cases due to lower risk of extrapyramidal side effects (41). Consensus has not been reached as to their effectiveness or superiority to haloperidol to control dangerous POD symptoms (9,37,50). Before administering antipsychotics, it is important to perform an electrocardiogram because antipsychotics can cause QTc prolongation. Thus for patients with prolonged QTc or Parkinsonism, antipsychotics are contraindicated (41). Further, benzodiazepines can exacerbate delirium and should be avoided in patients with POD unless the primary etiology of POD is benzodiazepine withdrawal (41).
Impact
The impact of POD can be felt well beyond a patient’s postoperative admission. Patients who experience POD have lower cognitive function as measured by the Mini Mental State Examination one month after surgery and as long as 1 year after surgery (51). In addition, as many as one-third of patients with POD who require admission to a post-acute care facility after surgery show continued symptoms of delirium six months after their operations (52). These patients with persistent delirium have a higher rate of 1-year mortality (52) and lower rate of functional recovery than those whose POD resolved (53).
Treatment of POD requires substantial healthcare resources, with average cumulative costs of >$40,000 per patient (6). These costs are attributable to delirium alone and not to the surgical treatment that prompted POD. This metric allows us to extrapolate to a cost of >$32 billion per year to treat an estimated 700,000 patients with POD in the United States (6). These costs are the result of longer lengths of postoperative hospital stay, more frequent complications and readmissions, and long-term functional decline (7,54). In addition to financial costs is the impact of POD on families—patients often do not recover their baseline levels of physical or cognitive function and require substantial assistive care.
Conclusions
POD is the most common postoperative complication in older adults and can occur at any time after a patient’s operation. Risk factors for developing POD include age, malnutrition, baseline cognitive dysfunction, anemia, pain, lack of mobilization, and disruption of sleep/wake cycles. POD is often diagnosed with CAM and treated with frequent patient re-orientation as well as helping patients return to their normal daily routines. Antipsychotic medications can be helpful for symptom management in patients who experience uncontrolled agitation that poses a danger to self or others but should not be used for patients without these symptoms. Ultimately, the best form of treatment is prevention, which can be accomplished through standardized behavioral interventions carried out by multidisciplinary care teams.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Video-Assisted Thoracic Surgery for the series “VATS in Older Adults”. The article has undergone external peer review.
Peer Review File: Available at https://vats.amegroups.com/article/view/10.21037/vats-22-23/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-22-23/coif). The series “VATS in Older Adults” was commissioned by the editorial office without any funding or sponsorship. MRK is Chair of the Standards and Verification Committee of the American College of Surgeons Geriatric Surgery Verification Program and was part of the program’s Core Development Group; he also served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from April 2022 to March 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Joosten E, Lemiengre J, Nelis T, et al. Is anaemia a risk factor for delirium in an acute geriatric population? Gerontology 2006;52:382-5. [Crossref] [PubMed]
- Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg 2009;249:173-8. [Crossref] [PubMed]
- van der Zanden V, Beishuizen SJ, Scholtens RM, et al. The Effects of Blood Transfusion on Delirium Incidence. J Am Med Dir Assoc 2016;17:748-53. [Crossref] [PubMed]
- Inouye SK, Ferrucci L. Elucidating the pathophysiology of delirium and the interrelationship of delirium and dementia. J Gerontol A Biol Sci Med Sci 2006;61:1277-80. [Crossref] [PubMed]
- Becher RD, Wyk BV, Leo-Summers L, et al. The Incidence and Cumulative Risk of Major Surgery in Older Persons in the United States. Ann Surg 2021; [Crossref]
- Gou RY, Hshieh TT, Marcantonio ER, et al. One-Year Medicare Costs Associated With Delirium in Older Patients Undergoing Major Elective Surgery. JAMA Surg 2021;156:430-42. [Crossref] [PubMed]
- Katlic MR, Robinson TN. The Costs of Postoperative Delirium. JAMA Surg 2021;156:470-1. [Crossref] [PubMed]
- Kang T, Park SY, Lee JH, et al. Incidence & Risk Factors of Postoperative Delirium After Spinal Surgery in Older Patients. Sci Rep 2020;10:9232. [Crossref] [PubMed]
- Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg 2011;112:1202-11. [Crossref] [PubMed]
- Schmitt EM, Marcantonio ER, Alsop DC, et al. Novel risk markers and long-term outcomes of delirium: the successful aging after elective surgery (SAGES) study design and methods. J Am Med Dir Assoc 2012;13:818.e1-10. [Crossref] [PubMed]
- Dyer CB, Ashton CM, Teasdale TA. Postoperative delirium. A review of 80 primary data-collection studies. Arch Intern Med 1995;155:461-5. [Crossref] [PubMed]
- Wang CG, Qin YF, Wan X, et al. Incidence and risk factors of postoperative delirium in the elderly patients with hip fracture. J Orthop Surg Res 2018;13:186. [Crossref] [PubMed]
- Chung KS, Lee JK, Park JS, et al. Risk factors of delirium in patients undergoing total knee arthroplasty. Arch Gerontol Geriatr 2015;60:443-7. [Crossref] [PubMed]
- Gleason LJ, Schmitt EM, Kosar CM, et al. Effect of Delirium and Other Major Complications on Outcomes After Elective Surgery in Older Adults. JAMA Surg 2015;150:1134-40. [Crossref] [PubMed]
- Jones RN, Marcantonio ER, Saczynski JS, et al. Preoperative Cognitive Performance Dominates Risk for Delirium Among Older Adults. J Geriatr Psychiatry Neurol 2016;29:320-7. [Crossref] [PubMed]
- Cizginer S, Marcantonio E, Vasunilashorn S, et al. The Cognitive Reserve Model in the Development of Delirium: The Successful Aging After Elective Surgery Study. J Geriatr Psychiatry Neurol 2017;30:337-45. [Crossref] [PubMed]
- Ravi B, Pincus D, Choi S, et al. Association of Duration of Surgery With Postoperative Delirium Among Patients Receiving Hip Fracture Repair. JAMA Netw Open 2019;2:e190111. [Crossref] [PubMed]
- Behrends M, DePalma G, Sands L, et al. Association between intraoperative blood transfusions and early postoperative delirium in older adults. J Am Geriatr Soc 2013;61:365-70. [Crossref] [PubMed]
- Lynch EP, Lazor MA, Gellis JE, et al. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg 1998;86:781-5. [Crossref] [PubMed]
- Leung JM, Sands LP, Lim E, et al. Does preoperative risk for delirium moderate the effects of postoperative pain and opiate use on postoperative delirium? Am J Geriatr Psychiatry 2013;21:946-56. [Crossref] [PubMed]
- Bitsch M, Foss N, Kristensen B, et al. Pathogenesis of and management strategies for postoperative delirium after hip fracture: a review. Acta Orthop Scand 2004;75:378-89. [Crossref] [PubMed]
- Bilotta F, Lauretta MP, Borozdina A, et al. Postoperative delirium: risk factors, diagnosis and perioperative care. Minerva Anestesiol 2013;79:1066-76.
- Mueller A, Spies CD, Eckardt R, et al. Anticholinergic burden of long-term medication is an independent risk factor for the development of postoperative delirium: A clinical trial. J Clin Anesth 2020;61:109632. [Crossref] [PubMed]
- Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA 1994;272:1518-22.
- Janjua MS, Spurling BC, Arthur ME. Postoperative Delirium. StatPearls Publishing; 2022 Jan.
- Milisen K, Foreman MD, Wouters B, et al. Documentation of delirium in elderly patients with hip fracture. J Gerontol Nurs 2002;28:23-9. [Crossref] [PubMed]
- Robinson TN, Raeburn CD, Tran ZV, et al. Motor subtypes of postoperative delirium in older adults. Arch Surg 2011;146:295-300. [Crossref] [PubMed]
- Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry 2000;5:75-85. [Crossref] [PubMed]
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014;383:911-22. [Crossref] [PubMed]
- Wingfield S. Postoperative delirium in seniors: Recognizing the symptoms, reducing the risks. Aging | Brain | UT Southwestern Medical Center. UT Southwestern Medical Center MedBlog. 2020. Accessed June 7, 2022. Available online: https://utswmed.org/medblog/postoperative-delirium-seniors-recognizing-symptoms-reducing-risks/
- Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338-44. [Crossref] [PubMed]
- Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941-8. [Crossref] [PubMed]
- Marcantonio ER, Ngo LH, O'Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med 2014;161:554-61. [Crossref] [PubMed]
- Vasunilashorn SM, Guess J, Ngo L, et al. Derivation and Validation of a Severity Scoring Method for the 3-Minute Diagnostic Interview for Confusion Assessment Method--Defined Delirium. J Am Geriatr Soc 2016;64:1684-9. [Crossref] [PubMed]
- Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703-10. [Crossref] [PubMed]
- HealthInAging.org. Ask The Expert: Prevention And Treatment Of Post-Operative Delirium. 2019. Accessed June 2, 2022. Available online: https://www.healthinaging.org/tools-and-tips/ask-expert-prevention-and-treatment-post-operative-delirium
- Flinn DR, Diehl KM, Seyfried LS, et al. Prevention, diagnosis, and management of postoperative delirium in older adults. J Am Coll Surg 2009;209:261-8; quiz 294. [Crossref] [PubMed]
- Hayhurst CJ, Pandharipande PP, Hughes CG. Intensive Care Unit Delirium: A Review of Diagnosis, Prevention, and Treatment. Anesthesiology 2016;125:1229-41. [Crossref] [PubMed]
- Sheikh AB, Javed N, Leyba K, et al. Pet-Assisted Therapy for Delirium and Agitation in Hospitalized Patients with Neurocognitive Impairment: A Review of Literature. Geriatrics (Basel) 2021;6:96. [Crossref] [PubMed]
- Cheong CY, Tan JA, Foong YL, et al. Creative Music Therapy in an Acute Care Setting for Older Patients with Delirium and Dementia. Dement Geriatr Cogn Dis Extra 2016;6:268-75. [Crossref] [PubMed]
- Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med 2003;163:2716-24. [Crossref] [PubMed]
- Segatore M. Managing the surgical orthopaedic patient with Parkinson's disease. Orthop Nurs 1998;17:13-20; quiz 21-2. [Crossref] [PubMed]
- Chen CC, Li HC, Liang JT, et al. Effect of a Modified Hospital Elder Life Program on Delirium and Length of Hospital Stay in Patients Undergoing Abdominal Surgery: A Cluster Randomized Clinical Trial. JAMA Surg 2017;152:827-34. [Crossref] [PubMed]
- Swarbrick CJ, Partridge JSL. Evidence-based strategies to reduce the incidence of postoperative delirium: a narrative review. Anaesthesia 2022;77:92-101. [Crossref] [PubMed]
- Chuan A, Zhao L, Tillekeratne N, et al. The effect of a multidisciplinary care bundle on the incidence of delirium after hip fracture surgery: a quality improvement study. Anaesthesia 2020;75:63-71. [Crossref] [PubMed]
- Program GSVQI. Optimal Resources for Geriatric Surgery: 2019 Standards. 2019. Available online: https://www.facs.org/media/f10eka54/geriatricsv_standards.pdf
- Jones TS, Jones EL, Richardson V, et al. Preliminary data demonstrate the Geriatric Surgery Verification program reduces postoperative length of stay. J Am Geriatr Soc 2021;69:1993-9. [Crossref] [PubMed]
- Girard TD, Exline MC, Carson SS, et al. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. N Engl J Med 2018;379:2506-16. [Crossref] [PubMed]
- Maldonado JR. Delirium in the acute care setting: characteristics, diagnosis and treatment. Crit Care Clin 2008;24:657-722. vii. [Crossref] [PubMed]
- Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev 2007;CD005594. [Crossref] [PubMed]
- Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med 2012;367:30-9. [Crossref] [PubMed]
- Kiely DK, Marcantonio ER, Inouye SK, et al. Persistent delirium predicts greater mortality. J Am Geriatr Soc 2009;57:55-61. [Crossref] [PubMed]
- Kiely DK, Jones RN, Bergmann MA, et al. Association between delirium resolution and functional recovery among newly admitted postacute facility patients. J Gerontol A Biol Sci Med Sci 2006;61:204-8. [Crossref] [PubMed]
- Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA 2012;308:73-81. [Crossref] [PubMed]
Cite this article as: Miller SM, Glerum KM, Jones RN, Yildiz F, Katlic MR, Vrees M, Cioffi W, Besdine RW, Cizginer S. Postoperative delirium in older adults: a surgeon’s guide and clinical practice review. Video-assist Thorac Surg 2022;7:25.


