
Laparoscopic paraesophageal hernia repair with absorbable mesh: a systematic review
Introduction
Hiatus hernia (HH) is a heterogeneous anatomic/clinical entity wherein abdominal viscera, most commonly the stomach, are dislocated across the esophageal hiatus. The current classification recognizes four types of HH. Type I (sliding HH) is associated with hiatal widening, laxity of the phrenoesophageal membrane and upward migration of the cardia. These are the most common HH type and are often associated with gastroesophageal reflux disease (GERD). Paraesophageal hernias (PEH) are less common types and approximately account for 5–15% of HH (1,2). Type II hernia consist in an upward herniation of the gastric fundus with a normally located gastroesophageal junction. Type III hernia has elements of both type I and II hernias with herniation of both fundus and displacement of the gastroesophageal junction above the diaphragm. Type IV HH is characterized by larger hiatal defects with upward migration of the stomach and other intra-abdominal organs or omentum. Although these hernias may be associated with GERD their clinical significance lies in the potential for mechanical complications, pulmonary impairment, and chronic bleeding (3).
Proper management and surgical indication of PEH is debated. Laparoscopic repair is the standard of care for symptomatic patients. Surgery is also recommended in asymptomatic patients with type III–IV hernia because the potential to develop related complications (4). Hernia recurrence is a puzzling problem with a reported incidence up to 60% (5-8). Since the first laparoscopic crural mesh reinforcement, different prosthetic materials have been proposed to bolster the hiatus in attempt to minimize recurrence (9). The ideal mesh material should be able to reinforce the hiatus and reduce crural tension without causing visceral erosion or dysphagia. While the use of non-absorbable mesh has been reported to be promising in term of recurrence minimization, recent studies questioned their safety profile because concerns of mesh-related complications (i.e., infection, migration, stenosis, esophageal/gastric erosion) (10-15). Opposite, absorbable mesh seems associated with mitigated related complications and similar short/medium-term hernia recurrence (16). Interestingly, a recent assessment by the Society of American Gastrointestinal Endoscopic Surgeons (SAGES) revealed that the majority of surgeon treating PEH preferred the use of absorbable mesh (17). Absorbable mesh can be both synthetic or biological with different technical/engineering characteristics, scaffold structure and resorption time (18,19). Nowadays, literature data reporting outcomes for absorbable mesh reinforced cruroplasty are sparse and puzzled.
Hence, the aim of this systematic review was to summarize current knowledge on laparoscopic PEH repair with absorbable mesh crural reinforcement. We present the following article in accordance with the PRISMA reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-22-27/rc) (20,21).
Methods
The present systematic review was not registered. Ethical approval was not required. PubMed (1949–present), MEDLINE (1946–present), EMBASE (1947–present), Scopus (2004–present), Google Scholar (2004–present), and ClinicalTrials.gov (2000–present) were executed (22,23). The last date of search was the May 31st, 2022. A combination of the following MeSH terms (Medical Subject Headings) was adopted (“hiatus hernia” (tiab), OR “hiatal hernia” (tiab)) AND (“mesh” (tiab), OR “reinforcement” (tiab)) AND (“hiatoplasty” (tiab), OR “cruroplasty” (tiab)) AND (“recurrence” (tiab), OR “reoperation” (tiab)) AND (“absorbable” (tiab), OR “resorbable” (tiab)) AND (“synthetic” (tiab), OR “biologic” (tiab)). Five authors (AA, AS, FL, AL, and CO) independently conducted the literature search and separately evaluated suitable titles, abstracts and cited references contained in every article. In case of disagreement among authors, a sixth senior author (GC) clarified discrepancies.
Eligibility criteria
Inclusion criteria: (I) cohort studies and randomized controlled trials (RCTs) reporting outcomes for elective laparoscopic PEH repair with cruroplasty and absorbable mesh reinforcement in adult patients (≥18 year old); (II) English written; (III) when two or more papers were published by the same institution or study group, articles with the longest follow-up or the largest sample; (IV) in case of duplicate studies with accumulating numbers of patients only the most complete reports were included. Exclusion criteria: (I) not English-written; (II) studies with follow-up shorter than 12 months; (III) articles with less than 10 patients per study arm; (IV) articles reporting data for open or non-absorbable mesh reinforced cruroplasty.
Data extraction
The following data were retrieved: authors, country, year of publication, design of the study, number of included patients, gender, age, body mass index (BMI), American Society of Anesthesiologists (ASA) physical status, comorbidities, surgical indication, type of surgical procedure, type of mesh (synthetic vs. biologic), follow-up and outcomes. All data were independently processed by five authors (AA, AS, FL, AL, and CO) and matched at the end of the revising process. A sixth author (DB) determined disagreements.
Quality assessment
Three authors (AE, VP, MC) judged the methodological quality of included studies with the ROBINS-I tool (24). Confounding bias, selection bias, classification bias, intervention bias, missing data bias, outcomes measurement bias, and reporting bias were pondered. Each domain was evaluated with one of the following: “yes”, “probably yes”, “probably no”, or “no”. The categories of judgement for each study are low, moderate, serious, and critical risk of bias. Incongruities were clarified.
Results
The PRISMA flow chart is reported in Figure 1. Overall, 1,065 publications were identified. After duplicates removal, 892 titles were screened, and 127 studies were found possibly relevant for full-text assessment. After full text evaluation, 39 studies (3,103 patients) meet the inclusion/exclusion criteria and were included in the systematic review (Table 1). Notably, 26 studies were of retrospective design, 10 were prospective while 3 were RCTs. The quality of included studies is summarized in Table S1.
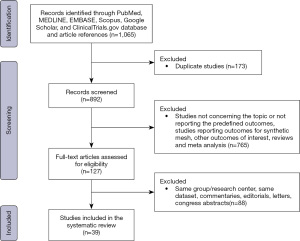
Table 1
| Study, year | Study design | No. of pts | Sex, F/M | Mean age (years) | BMI (kg/m2) | Type of mesh | Mesh shape | Fixation method | Antireflux procedure, N-T-O | OT (min) | MRC (No.) | Follow up (mos) | Recurrence (No.) | Redo surgery (No.) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synthetic mesh | Massullo et al., 2012, (25) | Ret | 11 | 9/2 | 60 [42–85] | 30.7 [21.9–42.5] | Bio-A® | U | AT | NR-NR-0 | NR | 0 | 13 [11.6–15.7] | 1 | NR |
| Powell et al., 2013, (26) | Ret | 70 | 47/23 | 50.7 | 28.3 | Bio-A® | U | Glue | 70-0-0 | NR | 0 | 12 | 0 | NR | |
| Priego Jiménez et al., 2014, (27) | Ret | 10 | 7/3 | 65.5 [53–82] | 31.65 [27.2–39.6] | Bio-A® | U | 6 AT; 4 AT + Glue | 10-0-0 | 162 [120–240] | 0 | 20.3 [10–30] | 1 | NR | |
| Alicuben et al., 2014, (28) | Ret | 114 | 65/49 | 66 | NR | Bio-A® | U | AT, AS or Glue | 76-38-0 | NR | 0 | 12 | 1 | 0 | |
| Silecchia et al., 2014, (29) | Pros | 10 | 9/1 | 52±9.3 | 26.4±2.4 | Bio-A® | U | AT + Glue | 10-0-0 | 70±11 | 0 | 17.4 | 0 | 0 | |
| Asti et al., 2016, (30) | Ret | 41 | 29/12 | 65.9±10.5 | 27.2±3.7 | Bio-A® | U | NAS | 7-34-0 | 175 [IQR 78] | 0 | 24 [IQR 29] | 4 | 0 | |
| Gebhart et al., 2013, (31) | Ret | 92 | 55/37 | 57.3±14.3 | NR | Bio-A® | U | NAS | 67-0-25 none | 88±25 | 0 | 30±11 | 17 | NR | |
| Olson et al., 2018, (32) | Pros | 399 | 261/138 | 59.6±13.4 | 29.9±5.0 | Bio-A® | U | AT | 225-170-4 Dor | NR | 1 esophageal stenosis | 44.7±22.8 | 49 | 24 | |
| Iossa et al., 2019, (33) | Ret | 28 | 18/10 | 46±23 | 23±5 | Bio-A® | U | NR | 28-0-0 | 90±13 | 0 | 41 [17–51] | 2 | 0 | |
| Tartaglia et al., 2021, (34) | Ret | 44 | 29/15 | 62 [18–85] | 24.5 [21–29] | Bio-A® | U | AS | 26-18-0 | 127 [99–150] | 0 | 36 | 2 | 0 | |
| Abdelmoaty et al., 2020, (35) | Pros | 50 | 32/18 | 67 [44–84] | 30.6 [20–41.5] | Phasix-ST® | U | 24 pledgets; 26 AT + pledgets | 17-33-0 | 161 | 0 | 12 | 4 | 0 | |
| Panici Tonucci et al., 2020, (36) | Ret | 73 | 47/26 | 68.2±23 | 26.9±3.5 | Phasix-ST® | U | MC | 0-73-0 | NR | 0 | 17 [9–24] | 2 | 0 | |
| Aiolfi et al., 2022, (37) | Ret | 68 | 54/14 | 66.3±12.7 | 26.3±5.1 | Phasix-ST® | U | AS | 0-68-0 | 148 [96–188] | 0 | 27 [1–53] | 6 | 0 | |
| Zehetner et al., 2010, (38) | Ret | 35 | 25/10 | 70 [48–89] | 30.4 [20.4–44.8] | Vicryl® | NR | Bio Glue | 35-0-0 | 144 [101–311] | 0 | 14 [11–34] | 2 | 0 | |
| Parsak et al., 2011, (39) | RCT | 75 | 33/42 | 48.4±11 | 27.4±5.5 | Vicryl® | U | AT | 75-0-0 | 65 [40–110] | 0 | 36.1±15 | 3 | 0 | |
| Reynolds et al., 2016, (40) | Ret | 190 | 132/58 | 68 [36–93] | NR | Vicryl® | Y | Bio Glue | NR-NR-0 | NR | 0 | 24.5 [12–88] | 12 | NR | |
| Biologic mesh | Wisbach et al., 2006, (41) | Pros | 11 | 2/9 | 41 [26–60] | NR | AlloDerm® | Y | AT | 11-0-0 | NR | 0 | 12 [8–19] | 0 | NR |
| Lee E et al., 2007, (42) | Ret | 17 | 13/4 | 65±12 [45–85] | 31±4 | AlloDerm® | U | AS | 17-0-0 | 273±48 | 0 | 14.4±4.4 | 1 | 0 | |
| Lee YK et al., 2008, (43) | Ret | 52 | 28/24 | 56.7 [34–74] | NR | AlloDerm® | U | AS | 52-0-0 | 121 [75–235] | 0 | 16 [12–24] | 2 | 1 | |
| Bell et al., 2013, (44) | Ret | 252 | 164/88 | 57±13.4 | 30±5.7 | AlloDerm® | 52 U 140 C | AS | 224-28-0 | NR | 0 | 17.7 [6–51] | 24 | NR | |
| Shmidt et al., 2014, (45) | Ret | 38 | 21/17 | 51 | 31.4 | AlloDerm® | U | AS | NR | NR | 0 | 13.3 [1–42] | 0 | 0 | |
| Ward et al., 2015, (46) | Pros | 17 | 4/13 | 64.3±10 | 32.6±6.5 | AlloDerm® | U | NR | 17-0-0 | 244±29.8 | 0 | 30.7±13 | 3 | 0 | |
| 37 | 6/31 | 60.8±10.5 | 31.3±4.7 | FlexHD® | U | NR | 37-0-0 | 214±34.4 | 0 | 29.5±13.4 | 5 | 4 | |||
| Rosen et al., 2019, (47) | Pros | 41 | 33/8 | 63.3±12.5 | 30.7±7.0 | Miromesh® | 39 U 2 C |
NAS | 20-11-1 Dor. 9 gastropexy | 142.6±45 | NR | 24 | 3 | 0 | |
| Antonakis et al., 2016, (48) | Pros | 10 | 7/3 | 73±13 [26–81] | NR | Permacol® | C | NAS + Glue | 10-0-0 | NR | 1 dysphagia due to dense fibrosis | 27±18 | 0 | 1 | |
| Lomelin et al., 2017, (49) | Ret | 35 | 26/9 | 63.1±12 | 30.8±6.3 | Strattice® | U | AS | 29-5-1 Dor | 147.8±29 | 0 | 12 | 5 | NR | |
| Shrestha et al., 2019, (50) | Ret | 30 | 22/8 | 70 [49–85] | 30 [23–39] | Strattice® | NR | NAS | 30-0-0 | 180 [120–510] | 0 | 50 [36–60] | 2 | 0 | |
| 30 | 24/6 | 71 [42–89] | 29 [19–42] | Veritas® | C | NAS | 30-0-0 | 180 [135–330] | 0 | 71 [60–84] | 2 | 1 | |||
| Jacobs et al., 2007, (51) | Ret | 93 | 52/41 | 47.4 | NR | Surgisis® | U | NAS | 78-15-0 | NR | 0 | 38 | 3 | NR | |
| Oelschlager et al., 2011, (52) | RCT | 26 | 20/6 | 64±10 | 31.1±5.8 | Surgisis® | U | AS | 26-0-0 | NR | NR | 58 [40–78] | 14 | 0 | |
| Wassenaar et al., 2012, (53) | Pros | 73 | 53/20 | 62.3±13.2 | 30.3±5.5 | Surgisis® | U | AS + Glue | 70-3-0 | NR | 0 | 12 | 3 | 1 | |
| Watson et al., 2015, (54) | RCT | 41 | 31/10 | 68 [65.1–70.9] | 29.4 [27.8–31] | Surgisis® | U | AT | NR | 110 [96.7–124] | 0 | 12 | 4 | 4 | |
| Wang B et al., 2016, (55) | Ret | 32 | 16/16 | NR | NR | Surgisis® | U | AS | NR | NR | 0 | 40 [37–49] | 6 | NR | |
| Korwar et al., 2019, (56) | Ret | 154 | 99/55 | 65±12 | NR | Surgisis® | U | NR | 148-6-0 | NR | 0 | 33.7±23 | 10 | 5 | |
| Nie et al., 2021, (57) | Ret | 36 | 24/12 | 68.4±17.2 | 28.6±6.8 | Thomal GEN® | U | Glue + AS | NR | 92.6 [73–135] | 0 | 18.4 [13–24] | 1 | 0 | |
| Wang CQ et al., 2019, (58) | Ret | 32 | 22/10 | 68±9.7 | NR | UBM | U | NAS | NR | 115 ±30 | 0 | 12 | 12 | 3 | |
| Sasse et al., 2016, (59) | Ret | 15 | 9/6 | 53 [27–72] | 34 [22–59] | UBM | U | AS | NR-NR-0 | 56 [36–136] | 0 | 37 [24–56] | 0 | 0 | |
| Lidor et al., 2015, (60) | Pros | 111 | 70/41 | 61.5±13.5 | NR | Veritas® | NR | NR | 111-0-0 | 314.5 | 0 | 19.9±16.4 | 19 | 1 | |
| Grimsley et al., 2022, (61) | Pros | 51 | 45/6 | 67±11 | 29.9±6.5 | UBM OR acellular bovine dermal collagen matrix | Key | NR | 22-24-1 None. 4 Dor | NR | 0 | 33±38 | 8 | 7 | |
| 58 | 46/12 | 67±12 | 29.6±4.5 | UBM OR acellular bovine dermal collagen matrix | Star | NR | 23-26-6 none. 3 MSA | NR | 0 | 33.3±38 | 11 | 6 | |||
| Multiple | Jones et al., 2015, (62) | Ret | 159 | 98/61 | 57.6±14.4 | 30.0±5.3 | AlloDerm® | NR | NR | 149-9-1 | NR | 0 | 25 [0–101] | 32 | NR |
| 35 | 8/27 | 57.6±14.4 | 30.0±5.3 | Bio-A® | NR | NR | 28-6-1 | NR | 0 | 25 [0–101] | 2 | NR | |||
| 15 | 14/1 | 57.6±14.4 | 30.0±5.3 | Strattice® | NR | NR | 10-4-1 | NR | 0 | 25 [0–101] | 1 | NR | |||
| Armijo et al., 2021, (63) | Ret | 162 | 66/96 | 60 [49–69] | 29.44 [26.8–32.3] | Human tissue matrix | U | NAS | 149-NR-NR | 157 [90–244] | 0 | 27 [1–166] | 67 | NR | |
| 83 | 37/46 | 57 [48–66] | 28.61 [26–31.16] | Bio-A® | U | NAS | 53-NR-NR | 188 [90–382] | 0 | 27 [1–166] | 30 | NR | |||
| 47 | 10/37 | 62 [58–74] | 29.7 [25.8–34] | Porcine tissue matrix | U | NAS | 38-NR-NR | 198.5 [91–439] | 0 | 27 [1–166] | 17 | NR |
Data are presented as mean ± SD, median [range] or numbers. pts, patients; F, females; M, males; BMI, body mass index; N, Nissen fundoplication; T, Toupet fundoplication; O, other procedures; OT, operative time; MRC, mesh-related complications; mos, months; Ret, retrospective; Pros, prospective; RCT, randomized controlled trial; UBM, urinary bladder matrix; U, U-shape; Y, Y-shape; C, circular shape; Key, keyhole shape; Star, starburst shape; NR, not reported; AT, absorbable tacks; AS, absorbable sutures; NAS, non-absorbable sutures; MC, metal clips; MSA, magnetic sphincter augmentation device.
The patient population ranged from 10 to 399 patients. The age ranged from 18 to 93 years old, 62.8% were females and the preoperative BMI ranged from 20 to 59 kg/m2. Hernia sac dissection and excision was reported in all studies. Posterior cruroplasty was performed in all included studies while anterior cruroplasty was reported in four studies. The most commonly reported mesh configuration was U-shape (83.7%), followed by circumferential (8.1%), keyhole (5.4%) and starburst (2.8%). Different methods for mesh fixation (sutures vs. fibrin glue vs. absorbable tacks) were adopted depending on operating surgeon preference and experience. Nissen (75.1%) and Toupet (21.1%) fundoplication were commonly performed while gastropexy was reported in two studies. The operative time ranged from 36 to 510 minutes.
The overall postoperative complication rate was 2.5%. Inadvertent intraoperative iatrogenic esophageal/gastric perforation related to viscera manipulation was reported in six patients (0.19%). Postoperative pulmonary complication rate was 1.83%; pneumonia, pneumothorax and pulmonary embolism were the most commonly reported complications. Postoperative cardiac complications occurred in 0.92% of patients and atrial fibrillation was commonly reported. Postoperative in-hospital mortality was 0.22%. Postoperative follow-up ranged from 12 to 166 months. Mesh-related complication rate was 0.06% with two patients reporting esophageal stricture related to dense visceral fibrosis (1 synthetic and 1 biologic mesh). No full-thickness erosions were reported. Hernia recurrence according to different definitions (Table S2) was diagnosed in 393 patients (12.7%) while re-do surgery for recurrence was required in 1.9% of patients. Postoperative dysphagia occurred in 158 patients (5.1%).
Discussion
The use of mesh to reinforce the hiatus is highly discussed with two recently published meta-analyses reporting no significant differences for simple suture cruroplasty versus cruroplasty reinforced with mesh (64,65). Nevertheless, some limitations and significant heterogeneity limit the validity and robustness of such studies. First, the definition of hernia recurrence, inclusion criteria, and surgical indications were heterogeneous. Second, surgeon experience, mesh materials, shape and crural fixation further contributed to inter-study heterogeneity. Finally, the follow-up was limited (up to 42 months). Therefore, a definitive and robust evidence-based indication is still to be defined. Our study group recently described a “patient-tailored algorithm” based on four measurable parameters (type of HH, hiatus diastasis, pillar tropism and recurrence) to decide if it is necessary to place or not a mesh to bolster the crural repair during laparoscopic PEH repair (66,67). This algorithm has been shown to be possibly valuable to assure procedure reproducibility, standardization, and uniformly interpret outcomes in a field where the decision to place or not the mesh is left to the operating surgeon “feeling of a weak crura” and experience. Nowadays, there is still a lack of consensus regarding the best mesh material for crural buttressing after repair. Given the potential for tissue ingrowth rather than encapsulation, absorbable meshes (synthetic and biologic) are generally preferred over non-absorbable meshes (68). Advantages include reduced perivisceral inflammation and consequent tissue fibrosis with minimization of related complications such as esophageal and gastric erosion, mesh migration, and visceral stenosis (13-15). Three absorbable synthetic meshes are currently available for laparoscopic PEH repair: Bio-A® (Gore Medical, Newark, DE, USA), Phasix® (Bard, Warwick, RI, USA) and Vicryl® (Ethicon, Somerville, NJ, USA).
The Bio-A® is an absorbable synthetic mesh made of 67% polyglycolic acid and 33% trimethylene carbonate (69). Specifically, the mesh acts as a scaffold for the network of cells related to the inflammatory response. During prosthesis absorption (up to 6 months), these cells progressively migrate into the interstice of the mesh with consequent synthesis of new collagen and connective tissue that gradually replace the mesh. Nowadays, there are a few published studies reporting outcomes with Bio-A®. Specifically, Massullo et al. reported their retrospective experience with 11 patients operated for PEH and managed with Nissen or Toupet fundoplication. Short-term outcomes (13-month follow-up) were encouraging with 9% recurrence rate and no reported mesh-related complications (25). Similarly, Powell et al. in their retrospective series described promising short-term outcomes (12 months) and no mesh-related complications (26). Similarly, Iossa et al. reported their medium-term results (42-month follow-up) on 120 patients with Bio-A®. Postoperative recurrence rate was 6.2% (33). Asti et al. described their retrospective experience with 100 patients operated for PEH with laparoscopic Toupet fundoplication. No mesh-related complications were observed, and the medium-term (30-month follow-up) recurrence rate was 9% (30). Olson et al. in their single-center experience, reported data for 399 patients. All patients underwent Nissen, Toupet or Dor fundoplication. Results in term of postoperative recurrence, need for reoperation and complications were assessed at 45-month follow-up. Overall, 7.9% of patients underwent reoperation while 16% of patients had symptom recurrence (32). Tartaglia et al. described outcomes for 44 patients with HH treated with laparoscopic fundoplication (Nissen and Toupet) and Bio-A®. Radiologic recurrence rate was 4.5% with no need for reoperation nor mesh related complications at 3-year follow-up (34). Interestingly, in all included studies a rectangular 7×10 cm Bio-A®, shaped into a “U” configuration, was placed over the closed hiatus, and fixed with absorbable tacks, fibrin glue or stitches depending on operating surgeon preference.
The Phasix® mesh is made of poly-4-hydroxybutyrate (P4HB), a naturally derived polymer. The P4HB degrades through both hydrolysis and a hydrolytic enzymatic digestive process in about 12–18 months (35). As described for the Bio-A®, the Phasix® mesh is progressively resorbed and gradually replaced with connective tissue synthetized by patient fibroblast that migrates into mesh interstices in the early phase. The first report describing outcomes for the Phasix® mesh was published by Abdelmoaty et al. in 2020. The authors reported their experience with 50 patients. Mean length of hospital stay was 2.8 days with no major morbidity nor mortality. On the 1-year follow-up, hernia recurrence rate was 8% with no need for reoperation nor mesh infection/erosion (35). Panici Tonucci et al. described their retrospective experience with 73 patients with PEH and Toupet fundoplication. Results were reported at 17 months median follow-up. Postoperative hernia recurrence rate was 3.2% with no mesh-related complications or need for reoperation (36). Similarly, a study from our study group reported the experience with 68 patients with laparoscopic PEH repair and Toupet fundoplication. The median follow-up time was 26 months (range, 1–52 months). Hernia recurrence rate was 8.8%. The recurrence-free probability at 34 and 60 months was 0.89 (95% CI: 0.807–0.988) and 0.86 (95% CI: 0.76–0.97), respectively. During follow-up, hernia recurrence was predominantly observed between 21 and 36 months. No mesh-related complications were detected. None of the patients required surgical revision and all were managed with proton pump inhibitors (PPI). Patient-related quality of life, measured with both the GERD-HRQL and SF-36 was significantly improved compared to baseline (37). As described for Bio-A®, most of the studies reported a U-shape mesh configuration fixed, over the closed hiatus, with different methods. Finally, a recent report by Konstantinidis et al. described the use of Phasix-ST® in 40 patients that underwent robotic PEH repair and Nissen fundoplication. Over a median follow-up of 21 months no recurrences nor mesh related complications were observed (70).
Polyglactin 910 mesh (Vycril®) is another absorbable mesh with a degradation time ranging from 6 to 8 weeks. Zehetner et al. published their experience with polyglactin mesh placed in 35 patients with PEH (38). At 1-year follow-up, recurrence rate was 9.5% with no mesh-related complications. Similarly, Parsak et al. published a randomized trial comparing crural reinforcement with polypropylene vs. Vycril® including 150 patients (75 polypropylene vs. 75 polyglactin) (39). Postoperative morbidity was similar for both groups, with no mesh-related complications. At 36-month mean follow-up the overall recurrence rate was 7.5%.
Biologic mesh was developed and introduced as alternative and substitute to non-absorbable synthetic mesh. They support hiatal repair during the early phase thus providing a temporary collagen matrix for native tissue ingrowth. Different types of biologic mesh have been produced. They are generally constituted by collagen matrix derived from human acellular cadaveric dermis, porcine small intestine submucosa, porcine dermal collagen, or bovine pericardium. A mild inflammatory response and neovascularization have been reported for biologic grafts (71,72). The theatre of biologic meshes is particularly heterogeneous therefore give an exhaustive overview is challenging. Surgisis® (Cook Medical, Bloomington, IN, USA), AlloDerm® (Allergan PLC, Dublin, Ireland) and Strattice® (Allergan PLC) are commonly used. Oelschlager et al. published in 2006 a trial comparing suture alone vs. Surgisis® reinforced cruroplasty for PEH. Overall, 108 patients with symptomatic PEH were included. At 6-month follow-up, there was a significantly reduced incidence of hernia recurrence in favor of Surgisis® (24% vs. 9%) (73). However, the medium-term follow-up analysis (58-month) showed no differences between the two groups in terms of hernia recurrence (59% vs. 54%) (52). Watson et al. compared outcomes between patients undergoing PEH repair by either synthetic (n=42) or biologic (n=41) mesh vs. patients with no mesh reinforcement (n=43). No significant differences were found at 6-month follow-up in term of hernia recurrence (21.8% vs. 23.1%; P=NS) (54). Lee et al. retrospectively reviewed their experience with AlloDerm® mesh (52 patients). At 16-month follow-up the recurrence rate was 3.8% with no mesh-related complications. Another recent experience from the same group consisted of a retrospective review of 35 patients treated with crural repair and Strattice® mesh. In the short-term follow-up (12-month) the recurrence rate was 14% (42,43). Finally, Lidor et al. described their experience with the Veritas mesh (Baxter International, IL, USA). At 12-month follow-up the postoperative recurrence rate was 27% with no mesh-related complications (60).
Intra and postoperative complications have been described. In the present study, intraoperative esophageal/gastric perforation was reported in six patients. This complication may be attributable to intraoperative difficulties in hernia reduction and visceral manipulation. Furthermore, operating surgeon inexperience, learning curve, improper traction of the gastric fundus/esophagogastric junction, and thermal injury may cause full thickness perforation (74). In case the perforation is immediately recognized, primary repair with interrupted sutures is advisable (75). The overall postoperative complication rate was 2.5% with pneumonia and pneumothorax commonly reported. Pneumonia may occur in patients with preoperative lung comorbidities or poor lung function therefore, a prompt postoperative pulmonary rehabilitation should be pursued (76). Pneumothorax generally occur because of inadvertent pleural injury during hernia sac dissection and excision. Surgeons should be aware of this potential complication while preventive trans-hiatal chest tube has been described in case of pleural injury (77,78). Mesh-related complications and postoperative dysphagia were reported in 0.06% and 5.1% of patients, respectively. The limited inflammatory response and minimized perivisceral fibrosis typical of all absorbable synthetic and biologic mesh may explain these findings.
Notably, there was a significant heterogeneity including indications for PEH repair, different types/sizes of HH, mesh configuration and shape (i.e., U-shape vs. keyhole vs. starburst, etc.), mesh position, diverse methods for mesh fixation and different type of fundoplication (i.e., Nissen vs. Toupet vs. Dor). In addition, some studies reported data for esophageal lengthening procedure (Collis gastroplasty) and/or diaphragmatic relaxing incisions. Finally, the definition of hernia recurrence (i.e., anatomical or radiological recurrence vs. >2 cm intrathoracic stomach in association with recurrent symptoms) and duration of follow-up were different among studies. Therefore, this significant interstudy heterogeneity limits the robustness of any conclusions. Hence, a definitive indication on the best mesh absorbable mesh for crural reinforcement during laparoscopic PEH repair is still to be defined.
Conclusions
Laparoscopic PEH repair with crural buttressing using absorbable mesh (synthetic or biological) is gaining acceptance within the surgical community. Both synthetic and biologic mesh are safe and effective in the short- and medium-term with acceptable postoperative complications, minimized mesh-related complications and acceptable recurrence rates. The safety and efficacy profile in the long-run mandates future well-designed studies. Focused trials are necessary to appraise the best absorbable mesh for crural buttressing thus possibly defining a treatment algorithm to guide surgeons in the choice of the most appropriate mesh material.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-22-27/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-22-27/coif). AA serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from June 2022 to May 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kahrilas PJ, Kim HC, Pandolfino JE. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol 2008;22:601-16. [Crossref] [PubMed]
- Landreneau RJ, Del Pino M, Santos R. Management of paraesophageal hernias. Surg Clin North Am 2005;85:411-32. [Crossref] [PubMed]
- Aiolfi A, Cavalli M, Sozzi A, et al. Paraesophageal hernia repair with laparoscopic Toupet fundoplication: impact on pulmonary function, respiratory symptoms and quality of life. Hernia 2022;26:1679-85. [Crossref] [PubMed]
- Kohn GP, Price RR, DeMeester SR, et al. Guidelines for the management of hiatal hernia. Surg Endosc 2013;27:4409-28. [Crossref] [PubMed]
- Hashemi M, Peters JH, DeMeester TR, et al. Laparoscopic repair of large type III hiatal hernia: objective followup reveals high recurrence rate. J Am Coll Surg 2000;190:553-60; discussion 560-1. [Crossref] [PubMed]
- Dallemagne B, Kohnen L, Perretta S, et al. Laparoscopic repair of paraesophageal hernia. Long-term follow-up reveals good clinical outcome despite high radiological recurrence rate. Ann Surg 2011;253:291-6. [Crossref] [PubMed]
- Saad AR, Velanovich V. Anatomic Observation of Recurrent Hiatal Hernia: Recurrence or Disease Progression? J Am Coll Surg 2020;230:999-1007. [Crossref] [PubMed]
- Armijo PR, Pokala B, Misfeldt M, et al. Predictors of Hiatal Hernia Recurrence After Laparoscopic Anti-reflux Surgery with Hiatal Hernia Repair: a Prospective Database Analysis. J Gastrointest Surg 2019;23:696-701. [Crossref] [PubMed]
- Rausa E, Manfredi R, Kelly ME, et al. Prosthetic Reinforcement in Hiatal Hernia Repair, Does Mesh Material Matter? A Systematic Review and Network Meta-Analysis. J Laparoendosc Adv Surg Tech A 2021;31:1118-23. [Crossref] [PubMed]
- Griffith PS, Valenti V, Qurashi K, et al. Rejection of goretex mesh used in prosthetic cruroplasty: a case series. Int J Surg 2008;6:106-9. [Crossref] [PubMed]
- Stadlhuber RJ, Sherif AE, Mittal SK, et al. Mesh complications after prosthetic reinforcement of hiatal closure: a 28-case series. Surg Endosc 2009;23:1219-26. [Crossref] [PubMed]
- Tatum RP, Shalhub S, Oelschlager BK, et al. Complications of PTFE mesh at the diaphragmatic hiatus. J Gastrointest Surg 2008;12:953-7. [Crossref] [PubMed]
- Li J, Cheng T. Mesh erosion after hiatal hernia repair: the tip of the iceberg? Hernia 2019;23:1243-52. [Crossref] [PubMed]
- Spiro C, Quarmby N, Gananadha S. Mesh-related complications in paraoesophageal repair: a systematic review. Surg Endosc 2020;34:4257-80. [Crossref] [PubMed]
- Sánchez-Pernaute A, Pérez-Aguirre ME, Jiménez AP, et al. Intraluminal mesh erosion after prosthetic hiatoplasty: incidence, management, and outcomes. Dis Esophagus 2019;32:doy131. [Crossref] [PubMed]
- Berselli M, Livraghi L, Latham L, et al. Laparoscopic repair of voluminous symptomatic hiatal hernia using absorbable synthetic mesh. Minim Invasive Ther Allied Technol 2015;24:372-6. [Crossref] [PubMed]
- Pfluke JM, Parker M, Bowers SP, et al. Use of mesh for hiatal hernia repair: a survey of SAGES members. Surg Endosc 2012;26:1843-8. [Crossref] [PubMed]
- Quesada BM, Coturel AE. Use of absorbable meshes in laparoscopic paraesophageal hernia repair. World J Gastrointest Surg 2019;11:388-94. [Crossref] [PubMed]
- Westcott LZ, Ward MA. Techniques for closing the hiatus: mesh, pledgets and suture techniques. Ann Laparosc Endosc Surg 2020;5:16.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Goossen K, Tenckhoff S, Probst P, et al. Optimal literature search for systematic reviews in surgery. Langenbecks Arch Surg 2018;403:119-29. [Crossref] [PubMed]
- Bona D, Micheletto G, Bonitta G, et al. Does C-reactive Protein Have a Predictive Role in the Early Diagnosis of Postoperative Complications After Bariatric Surgery? Systematic Review and Bayesian Meta-analysis. Obes Surg 2019;29:3448-56. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Massullo JM, Singh TP, Dunnican WJ, et al. Preliminary study of hiatal hernia repair using polyglycolic acid: trimethylene carbonate mesh. JSLS 2012;16:55-9. [Crossref] [PubMed]
- Powell BS, Wandrey D, Voeller GR. A technique for placement of a bioabsorbable prosthesis with fibrin glue fixation for reinforcement of the crural closure during hiatal hernia repair. Hernia 2013;17:81-4. [Crossref] [PubMed]
- Priego Jiménez P, Salvador Sanchís JL, Angel V, et al. Short-term results for laparoscopic repair of large paraesophageal hiatal hernias with Gore Bio A® mesh. Int J Surg 2014;12:794-7. [Crossref] [PubMed]
- Alicuben ET, Worrell SG, DeMeester SR. Resorbable biosynthetic mesh for crural reinforcement during hiatal hernia repair. Am Surg 2014;80:1030-3.
- Silecchia G, Iossa A, Cavallaro G, et al. Reinforcement of hiatal defect repair with absorbable mesh fixed with non-permanent devices. Minim Invasive Ther Allied Technol 2014;23:302-8. [Crossref] [PubMed]
- Asti E, Lovece A, Bonavina L, et al. Laparoscopic management of large hiatus hernia: five-year cohort study and comparison of mesh-augmented versus standard crura repair. Surg Endosc 2016;30:5404-9. [Crossref] [PubMed]
- Gebhart A, Vu S, Armstrong C, et al. Initial outcomes of laparoscopic paraesophageal hiatal hernia repair with mesh. Am Surg 2013;79:1017-21.
- Olson MT, Singhal S, Panchanathan R, et al. Primary paraesophageal hernia repair with Gore® Bio-A® tissue reinforcement: long-term outcomes and association of BMI and recurrence. Surg Endosc 2018;32:4506-16. [Crossref] [PubMed]
- Iossa A, Silecchia G. Mid-term safety profile evaluation of Bio-A absorbable synthetic mesh as cruroplasty reinforcement. Surg Endosc 2019;33:3783-9. [Crossref] [PubMed]
- Tartaglia E, Cuccurullo D, Guerriero L, et al. The use of biosynthetic mesh in giant hiatal hernia repair: is there a rationale? A 3-year single-center experience. Hernia 2021;25:1355-61. [Crossref] [PubMed]
- Abdelmoaty WF, Dunst CM, Filicori F, et al. Combination of Surgical Technique and Bioresorbable Mesh Reinforcement of the Crural Repair Leads to Low Early Hernia Recurrence Rates with Laparoscopic Paraesophageal Hernia Repair. J Gastrointest Surg 2020;24:1477-81. [Crossref] [PubMed]
- Panici Tonucci T, Asti E, Sironi A, et al. Safety and Efficacy of Crura Augmentation with Phasix ST Mesh for Large Hiatal Hernia: 3-Year Single-Center Experience. J Laparoendosc Adv Surg Tech A 2020;30:369-72. [Crossref] [PubMed]
- Aiolfi A, Cavalli M, Sozzi A, et al. Medium-term safety and efficacy profile of paraesophageal hernia repair with Phasix-ST® mesh: a single-institution experience. Hernia 2022;26:279-86. [Crossref] [PubMed]
- Zehetner J, Lipham JC, Ayazi S, et al. A simplified technique for intrathoracic stomach repair: laparoscopic fundoplication with Vicryl mesh and BioGlue crural reinforcement. Surg Endosc 2010;24:675-9. [Crossref] [PubMed]
- Parsak CK, Erel S, Seydaoglu G, et al. Laparoscopic antireflux surgery with polyglactin (vicryl) mesh. Surg Laparosc Endosc Percutan Tech 2011;21:443-9. [Crossref] [PubMed]
- Reynolds JL, Zehetner J, Bildzukewicz N, et al. A Durable Laparoscopic Technique for the Repair of Large Paraesophageal Hernias. Am Surg 2016;82:911-5.
- Wisbach G, Peterson T, Thoman D. Early results of the use of acellular dermal allograft in type III paraesophageal hernia repair. JSLS 2006;10:184-7.
- Lee E, Frisella MM, Matthews BD, et al. Evaluation of acellular human dermis reinforcement of the crural closure in patients with difficult hiatal hernias. Surg Endosc 2007;21:641-5. [Crossref] [PubMed]
- Lee YK, James E, Bochkarev V, et al. Long-term outcome of cruroplasty reinforcement with human acellular dermal matrix in large paraesophageal hiatal hernia. J Gastrointest Surg 2008;12:811-5.
- Bell RC, Fearon J, Freeman KD. Allograft dermal matrix hiatoplasty during laparoscopic primary fundoplication, paraesophageal hernia repair, and reoperation for failed hiatal hernia repair. Surg Endosc 2013;27:1997-2004. [Crossref] [PubMed]
- Schmidt E, Shaligram A, Reynoso JF, et al. Hiatal hernia repair with biologic mesh reinforcement reduces recurrence rate in small hiatal hernias. Dis Esophagus 2014;27:13-7. [Crossref] [PubMed]
- Ward KC, Costello KP, Baalman S, et al. Effect of acellular human dermis buttress on laparoscopic hiatal hernia repair. Surg Endosc 2015;29:2291-7. [Crossref] [PubMed]
- Rosen MJ, Borao FJ, Binenbaum SJ, et al. A multi-center, prospective clinical trial of a hepatic derived porcine surgical mesh for the laparoscopic repair of symptomatic paraesophageal hernias. Am J Surg 2019;218:315-22. [Crossref] [PubMed]
- Antonakis F, Köckerling F, Kallinowski F. Functional Results after Repair of Large Hiatal Hernia by Use of a Biologic Mesh. Front Surg 2016;3:16. [Crossref] [PubMed]
- Lomelin D, Smith A, Bills N, et al. Long-Term Effectiveness of Strattice in the Laparoscopic Closure of Paraesophageal Hernias. Surg Innov 2017;24:259-63. [Crossref] [PubMed]
- Shrestha AK, Joshi M, DeBono L, et al. Laparoscopic repair of type III/IV giant para-oesophageal herniae with biological prosthesis: a single centre experience. Hernia 2019;23:387-96.
- Jacobs M, Gomez E, Plasencia G, et al. Use of surgisis mesh in laparoscopic repair of hiatal hernias. Surg Laparosc Endosc Percutan Tech 2007;17:365-8. [Crossref] [PubMed]
- Oelschlager BK, Pellegrini CA, Hunter JG, et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg 2011;213:461-8. [Crossref] [PubMed]
- Wassenaar EB, Mier F, Sinan H, et al. The safety of biologic mesh for laparoscopic repair of large, complicated hiatal hernia. Surg Endosc 2012;26:1390-6. [Crossref] [PubMed]
- Watson DI, Thompson SK, Devitt PG, et al. Laparoscopic repair of very large hiatus hernia with sutures versus absorbable mesh versus nonabsorbable mesh: a randomized controlled trial. Ann Surg 2015;261:282-9. [Crossref] [PubMed]
- Wang B, Zhang W, Shan CX, et al. Long-term outcomes of cruroplasty reinforcement with composite versus biologic mesh for gastroesophageal reflux disease. Surg Endosc 2016;30:2865-72. [Crossref] [PubMed]
- Korwar V, Adjepong S, Pattar J, et al. Biological Mesh Repair of Paraesophageal Hernia: An Analysis of Our Outcomes. J Laparoendosc Adv Surg Tech A 2019;29:1446-50. [Crossref] [PubMed]
- Nie Y, Xiong Y, Guan L, et al. Laparoscopic fixation of biological mesh at hiatus with glue and suture during hiatal hernia repair. BMC Surg 2021;21:158. [Crossref] [PubMed]
- Wang CQ, Tran T, Montera B, et al. Symptomatic, Radiological, and Quality of Life Outcome of Paraesophageal Hernia Repair With Urinary Bladder Extracellular Surgical Matrix: Comparison With Primary Repair. Surg Laparosc Endosc Percutan Tech 2019;29:182-6. [Crossref] [PubMed]
- Sasse KC, Warner DL, Ackerman E, et al. Hiatal Hernia Repair with Novel Biological Graft Reinforcement. JSLS 2016;20:e2016. [Crossref] [PubMed]
- Lidor AO, Steele KE, Stem M, et al. Long-term quality of life and risk factors for recurrence after laparoscopic repair of paraesophageal hernia. JAMA Surg 2015;150:424-31. [Crossref] [PubMed]
- Grimsley E, Capati A, Saad AR, et al. Novel "starburst" mesh configuration for paraesophageal and recurrent hiatal hernia repair: comparison with keyhole mesh configuration. Surg Endosc 2022; Epub ahead of print. [Crossref]
- Jones R, Simorov A, Lomelin D, et al. Long-term outcomes of radiologic recurrence after paraesophageal hernia repair with mesh. Surg Endosc 2015;29:425-30. [Crossref] [PubMed]
- Armijo PR, Krause C, Xu T, et al. Surgical and clinical outcomes comparison of mesh usage in laparoscopic hiatal hernia repair. Surg Endosc 2021;35:2724-30. [Crossref] [PubMed]
- Petric J, Bright T, Liu DS, et al. Sutured Versus Mesh-augmented Hiatus Hernia Repair: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann Surg 2022;275:e45-51. [Crossref] [PubMed]
- Angeramo CA, Schlottmann F. Laparoscopic Paraesophageal Hernia Repair: To Mesh or not to Mesh. Systematic Review and Meta-analysis. Ann Surg 2022;275:67-72. [Crossref] [PubMed]
- Aiolfi A, Cavalli M, Saino G, et al. Laparoscopic posterior cruroplasty: a patient tailored approach. Hernia 2022;26:619-26. [Crossref] [PubMed]
- Aiolfi A, Sozzi A, Cavalli M, et al. Patient-tailored algorithm for laparoscopic cruroplasty standardization: comparison with hiatal surface area and medium-term outcomes. Langenbecks Arch Surg 2022;407:2537-45. [Crossref] [PubMed]
- Köckerling F, Alam NN, Antoniou SA, et al. What is the evidence for the use of biologic or biosynthetic meshes in abdominal wall reconstruction? Hernia 2018;22:249-69. [Crossref] [PubMed]
- Available online: https://www.goremedical.com/products/bioatissue; accessed on September 9th 2022.
- Konstantinidis H, Charisis C. Surgical treatment of large and complicated hiatal hernias with the new resorbable mesh with hydrogel barrier (Phasix™ ST): a preliminary study. J Robot Surg 2022; [Crossref]
- Kaleya RN. Evaluation of implant/host tissue interactions following intraperitoneal implantation of porcine dermal collagen prosthesis in the rat. Hernia 2005;9:269-76. [Crossref] [PubMed]
- Antoniou SA, Koch OO, Antoniou GA, et al. Mesh-reinforced hiatal hernia repair: a review on the effect on postoperative dysphagia and recurrence. Langenbecks Arch Surg 2012;397:19-27. [Crossref] [PubMed]
- Oelschlager BK, Pellegrini CA, Hunter J, et al. Biologic prosthesis reduces recurrence after laparoscopic paraesophageal hernia repair: a multicenter, prospective, randomized trial. Ann Surg 2006;244:481-90. [Crossref] [PubMed]
- Botha AJ, Di Maggio F. Management of complications after paraesophageal hernia repair. Ann Laparosc Endosc Surg 2021;6:38.
- Chirica M, Kelly MD, Siboni S, et al. Esophageal emergencies: WSES guidelines. World J Emerg Surg 2019;14:26. [Crossref] [PubMed]
- Sihvo EI, Salo JA, Räsänen JV, et al. Fatal complications of adult paraesophageal hernia: a population-based study. J Thorac Cardiovasc Surg 2009;137:419-24. [Crossref] [PubMed]
- Bonavina L, Asti E, Sironi A, et al. Hybrid and total minimally invasive esophagectomy: how I do it. J Thorac Dis 2017;9:S761-72. [Crossref] [PubMed]
- Bona D, Aiolfi A, Asti E, et al. Laparoscopic Toupet fundoplication for gastroesophageal reflux disease and hiatus hernia: proposal for standardization using the "critical view" concept. Updates Surg 2020;72:555-8. [Crossref] [PubMed]
Cite this article as: Aiolfi A, Sozzi A, Lombardo F, Lanzaro A, Panizzo V, Bonitta G, Ogliari C, Dell’Era A, Cavalli M, Campanelli G, Bona D. Laparoscopic paraesophageal hernia repair with absorbable mesh: a systematic review. Video-assist Thorac Surg 2022;7:26.


