Use of gastropexy for paraesophageal hernias—a narrative review
Introduction
Paraesophageal hernias (PEH) comprise 5–15% of all hiatal hernias (1). While frank strangulation, obstruction, ischemia, necrosis, and perforation represent the most urgent presentation of patients with PEH, many may be reportedly asymptomatic (2). Often even in these patients, a careful discussion generally reveals subtle complaints. Often this includes early obstructive symptoms manifested by early satiety, some level of dysphagia or regurgitation, mild dyspnea, as well as more concerning or underappreciated signs such as post-prandial epigastric or chest pain. A history of weight loss and dietary changes over time not self-attributed to their hernia is often elicited. Similarly, a diagnosis of idiopathic or unrecognized anemia is often present without previous association to a known PEH. A careful history may elucidate preceding and long-standing gastroesophageal reflux symptoms in some patients prior to onset of more obstructive signs. However, many do not endorse these findings, or provide histories consistent with medically well-controlled symptoms without significant diminishment in quality of life.
Gastropexy involves fixing the stomach to intraabdominal structures to prevent its re-herniation into the chest or volvulus. Historically this was performed for patients with acute presentation of gastric volvulus undergoing emergent surgery to minimize time under general anesthesia, or for those thought to be too frail to undergo more definitive repair. Analysis of outcomes from 1997 to 2010 showed that non-elective repair of PEH had almost two times greater odds of major complications even after adjusting for age and comorbidities (3). For a select population, minimizing time under anesthesia and extent of dissection may still prove prudent. However, over the past couple decades the morbidity associated with elective PEH repair has continued to improve, even with an increase in the comorbidities of the patients (3,4). While urgent indications remain, utilization of gastropexy during non-emergent hiatal hernia repair may be evolving in the current era as an adjunct or alternative to formal gastric gastrofundoplication with or without esophageal lengthening procedures (5). This may be of particular interest in patients with large hernias with symptoms primarily associated with overt or early obstructive physiology, and without significant history or symptoms of gastroesophageal reflux, as mentioned above. In this article, we will review various techniques of gastropexy and their use in different clinical contexts. We present the following article in accordance with the Narrative Review reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-21-40/rc).
Methods
Throughout the writing process, an extensive literature search was done for articles relating to history, technique and outcomes of gastropexy in various clinical settings. PubMed was the primary source for articles, all in English or with a full English translation available. The description and images of our current technique came from institutional records. An example search is shown in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 18 Jan 2021 |
| Databases and other sources searched | PubMed |
| Search terms used | “Gastropexy”; “Paraesophageal hernia”; “Technique” |
| Timeframe | All dates |
| Inclusion and exclusion criteria | Inclusion: English; review articles |
| Exclusion: no full English translation, small case series | |
| Selection process | Conducted independently by RL Levesque and ET Alicuben, selected based on relevance and quality |
Gastropexy without hiatal hernia repair
Classically, gastropexy without hiatal hernia repair is reserved as an urgent operative intervention on a sick or frail patient. As introduced by Boerema and Germs in 1955 (6), and with reports of its use published by Nissen in 1956 (7), this strategy potentially avoids the extended time and general anesthesia required for formal PEH repair. These initial descriptions were of patients in which the stomach was reduced into the abdomen with minimal hiatal sac dissection and the lesser curve was secured with multiple sutures to the anterior abdominal wall through the linea alba to the right of the laparotomy incision. According to Boerema, the aim was to put the esophagus and lesser curve under tension. While initial reports of the Boerema anterior gastropexy were promising (8), long-term follow-up demonstrated complaints of reflux symptoms in 60% of patients (9). This led to its decreased use as a strategy in non-emergent repair with subsequent studies highlighting the importance of steps not included in the Boerema operation including formal dissection and reduction of the hernia sac (10). Currently, gastropexy alone is generally advised only in urgent/emergent circumstances if the patient is deemed too unfit to tolerate the extended time needed for formal dissection and hiatal repair.
Similar strategies have been used in conjunction with laparoscopy to further minimize the morbidity imposed on already frail patients (11,12). Yates et al. described their experience in 11 patients with acute or chronic obstructive gastric volvulus and too many medical comorbidities for prolonged repair (13). The authors developed a technique to secure the greater curve of the stomach to the left crus and anterior abdominal wall with interrupted sutures. Initially a percutaneous endoscopic gastrostomy tube was used to secure the antrum, but a modification to move the ports 5 cm caudal allowed for suturing to continue onto the antrum allowing for a total suture gastropexy. Ten patients were discharged tolerating a soft diet with 1 patient dependent on tube feeding due to severe oropharyngeal dysphagia. At a median follow-up of 3 months, all had resolution of symptoms with no recurrent episodes of volvulus.
While technically feasible, long-term durability and impact on quality of life remain poorly studied. In one of the only studies to report on long-term subjective quality of life, Bruenderman and colleagues followed 26 patients that had undergone laparoscopic suture gastropexy primarily due to comorbid conditions (14). At 2 years after surgery, 88% reported major improvement or complete resolution in their symptoms. However, 62% continued to use antacid medications. Two patients required reoperation, one for recurrent volvulus and one for gastric perforation.
Another commonly described approach to gastropexy in the urgent setting is the use of percutaneous endoscopic gastrostomy (PEG) tube placement to fix the stomach to the abdominal wall. This strategy involves endoscopic or laparoscopic reduction of an intrathoracic stomach followed by placement of the PEG tube. Many advise placement of two PEG tubes at a distance from each other to eliminate the possibility of a large, dilated stomach folding on itself around a single tube. In general, placement of PEG tubes is simple and quick, and avoids the need to laparoscopically suture to the abdominal wall, which can be technically challenging. Success with this strategy has been reported in multiple series (15-17). In patients that recover, the PEG tubes can be removed leaving the stomach attached to the abdominal wall. Also, if the patient is not able to tolerate oral intake, enteric access has already been established.
Arevalo et al. described the use of T-fasteners in combination with laparoscopic reduction in 4 patients who presented with acute gastric volvulus with high operative risk (18). After reduction of the stomach, the esophagus was dissected free without complete mediastinal mobilization and anterior cruroplasty performed. Under direct visualization, the device is inserted into the stomach and the T-bar deployed. The suture is then pulled to bring the stomach to the abdominal wall and secured it in place. With this gastropexy performed, a gastrostomy tube was placed in 3 of the 4 patients. All patients survived to discharge and were free of obstructive symptoms in short-term follow-up.
PEH repair with fundoplication and gastropexy
Laparoscopic repair of hiatal hernia continues to be plagued by a relatively high recurrence rate in some series, and much attention has been placed on optimizing operative strategies to improve this risk. Gastropexy has been proposed as an adjunct to standard repair with fundoplication to keep the stomach intra-abdominal should the crural repair fail. Ponsky et al. presented a prospective series of 28 patients where an anterior gastropexy was combined with PEH repair and an anti-reflux procedure, most commonly a modified Toupet fundoplication (19). They reported no recurrences at 2 years. In a separate series by Diaz et al., 48 of 116 patients who had laparoscopic PEH repair with fundoplication (either full Nissen fundoplication or a posterior Toupet fundoplication) also had gastropexy performed with T-fasteners if organoaxial rotation was present (20). While outcomes were generally reported as pooled data, the authors found no difference in radiographic recurrences at 30 months between patients that had gastropexy performed versus those that did not (25% vs. 13%, P=0.08).
In the largest published series on repair with fundoplication and gastropexy, Poncet et al. described their experience in 89 patients who underwent laparoscopic PEH repair with fundoplication (either a total Nissen-Rosetti fundoplication or a 270-degree fundoplication in cases at risk for dysphagia), 74 of whom underwent an anterior gastropexy following hiatal closure, similar in technique to that described by Boerema (21). For the gastropexy, two sutures were placed, one in the fundus and one in the antrum, to attach the stomach to the abdominal wall. While this was initially performed only for patients with a shortened esophagus or large hiatus, the procedure became their standard practice in the later portion of their series. At a median follow-up of 40 months, there were 14 recurrences (15.7%), 4 of which occurred soon after the initial operation, and noted to be early in the experience of the authors. Of the patients with recurrences, 8 required reoperations.
PEH repair with gastropexy and without fundoplication
Long-term studies of Nissen fundoplication have revealed durable control of reflux symptoms, but also a significant rate of complaints of dysphagia, and other sequelae including gas-bloat, diarrhea, and abdominal cramping (22). In a review of 187 patients undergoing laparoscopic PEH repair with fundoplication, we found that although this offered a durable repair and an improvement in quality of life scores, up to a quarter of patients reported dysphagia postoperatively (23). This has led to the reconsideration of the need for fundoplication in all patients, and specifically those whose history is without significant or uncontrolled reflux signs and/or symptoms, but with predominantly obstructive complaints (24) (often subtle and elicited through specific and thorough history taking). In this common clinical scenario, correcting the hiatal hernia and restoring near-normal gastric anatomy may prove an effective strategy to simultaneously relieve acute and/or chronic obstructive physiology, while also minimizing associated unwanted sequelae of gastrofundoplication in patients with an otherwise minimal and/or well-controlled history of reflux disease.
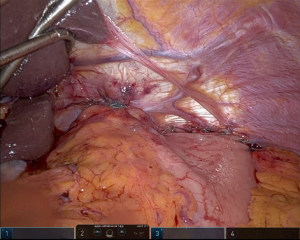
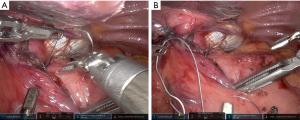
Our paradigm at the University of Pittsburgh has shifted over recent years to consider performing suture gastropexy in place of fundoplication in older and/or frail patients with primarily and/or exclusively obstructive symptoms. Importantly, the principal tenets of formal hernia reduction with high mediastinal mobilization, complete hernia sac reduction, and tension free repair of the hiatus are otherwise identical in all cases, with or without fundoplication (25). If gastropexy is elected in lieu of gastrofundoplication, a series of horizontal mattress sutures are placed along the line of the short gastric vessels to an everted fold of the left diaphragm approximating a line parallel to the anterior aspect of the spleen, thus grossly restoring the physiologic/anatomic position of the gastric fundus to the left upper quadrant (Figure 1). Of note, our technique has evolved to include an initial one to two sutures placed to reshape and reaffix the attenuated angle of His to the left crural pillar, theoretically providing some level of competence to the disrupted gastroesophageal junction/sphincter and disrupted phrenoesophageal apparatus (Figure 2). Overall, the goal is to refashion an intra-abdominal angle of His and restore the relative physiologic position of the stomach within the abdomen.
The efficacy of this strategy has been established with descriptions of patients without endoscopic evidence of esophagitis having undergone open repair of PEH combined with sutures to the diaphragm combined with anterior gastropexy. At a mean follow-up of 2.5 years, almost all patients had satisfactory symptom result. None of the patients developed esophagitis (26). Similar successful repairs have been reported for patients presenting with gastric volvulus including 1 patient with acute strangulation (27).
A multicenter study by Daigle et al. prospectively followed 101 patients undergoing PEH repair and anterior gastropexy (28). Patients with subjective or objective evidence of severe reflux were excluded. Dysphagia and recurrent emesis were the most common pre-operative symptoms. Following esophageal dissection and crural closure, 6 non-absorbable sutures were placed along the lesser curvature and tied to the anterior abdominal wall with aid of a suture passer. Per study protocol, 7 of the patients did not have crural closure due to poor tissue quality and/or significant tension. Seventy percent were without reflux symptoms at a median of 12 months follow-up. Only 9.9% of patients required daily proton pump inhibitor (PPI). They noted a recurrence rate of 16.8% with 6 of the 101 patients undergoing revisional surgery.
Conclusions
The use of gastropexy, while traditionally reserved in the emergent setting for patients presenting with signs of acute gastric volvulus, may be evolving as an option for patients undergoing elective or semi-elective repair of large PEH. While gastrofundoplication remains a standard approach and should certainly be considered in these cases, in patients presenting with predominantly obstructive signs and a history consistent with minimal or well-controlled reflux disease, gastropexy may provide significant clinical benefit while diminishing the less desirable known potential sequelae and/or side-effects of fundoplication. This may be especially true in both frail patients with multiple competing comorbidities, as well as those with otherwise well-preserved quality of life in terms of antecedent reflux symptomatology. At the University of Pittsburgh, our anecdotal experience with this selective approach has been positive. However, additional research and data are necessary regarding the clinical and quality of life outcomes in those with PEH repair with gastropexy vs repair with fundoplication. Formal review of our outcomes with these procedures is in process as we hope to quantify our experience and elucidate preoperative characteristics that are in favor of gastropexy vs. fundoplication during PEH repair.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Rishindra M. Reddy) for the series “Paraesophageal Hiatal Hernia Repairs, Transthoracic, Transabdominal, Laparoscopic, or Robotic, Which Method is Best” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-21-40/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-21-40/coif). The series “Paraesophageal Hiatal Hernia Repairs, Transthoracic, Transabdominal, Laparoscopic, or Robotic, Which Method is Best” was commissioned by the editorial office without any funding or sponsorship. JDL reports that he received consulting fees from Medtronic and honoraria from Covidien and owns stock in United Therapeutics, Cigna, Intuitive Surgical, Inc., and Smith & Nephew; and serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from April 2022 to March 2024. ISS reports that he received consulting fees from CMR, Auris, and Intuitive Surgical, honoraria from Intuitive Surgical, Boston Scientific, and VTI, and participation on Data Safety Monitoring Board for On Target Laboratories Phase 3 trial. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Buenaventura PO, Schauer PR, Keenan RJ, et al. Laparoscopic repair of giant paraesophageal hernia. Semin Thorac Cardiovasc Surg 2000;12:179-85. [Crossref] [PubMed]
- Schieman C, Grondin SC. Paraesophageal hernia: clinical presentation, evaluation, and management controversies. Thorac Surg Clin 2009;19:473-84. [Crossref] [PubMed]
- Tam V, Luketich JD, Winger DG, et al. Non-Elective Paraesophageal Hernia Repair Portends Worse Outcomes in Comparable Patients: a Propensity-Adjusted Analysis. J Gastrointest Surg 2017;21:137-45. [Crossref] [PubMed]
- Luketich JD, Nason KS, Christie NA, et al. Outcomes after a decade of laparoscopic giant paraesophageal hernia repair. J Thorac Cardiovasc Surg 2010;139:395-404, 404.e1.
- Kohn GP, Price RR, DeMeester SR, et al. Guidelines for the management of hiatal hernia. Surg Endosc 2013;27:4409-28. [Crossref] [PubMed]
- Boerema I, Germs R. Fixation of the lesser curvature of the stomach to the anterior abdominal wall after reposition of the hernia through the oesophageal hiatus. Arch Chir Neerl 1955;7:351-9. [PubMed]
- Nissen R. Gastropexy as the lone procedure in the surgical repair of hiatus hernia. Am J Surg 1956;92:389-92. [Crossref] [PubMed]
- Boerema WJ. Anterior gastropexy: a simple operation for hiatus hernia. Aust N Z J Surg 1969;39:173-5. [Crossref] [PubMed]
- Davies CJ. A survey of the results of the Boerema anterior gastropexy for hiatus hernia over a 4-year period. Br J Surg 1975;62:19-22. [Crossref] [PubMed]
- van der Peet DL, Klinkenberg-Knol EC, Alonso Poza A, et al. Laparoscopic treatment of large paraesophageal hernias: both excision of the sac and gastropexy are imperative for adequate surgical treatment. Surg Endosc 2000;14:1015-8. [Crossref] [PubMed]
- Agwunobi AO, Bancewicz J, Attwood SE. Simple laparoscopic gastropexy as the initial treatment of paraoesophageal hiatal hernia. Br J Surg 1998;85:604-6. [Crossref] [PubMed]
- Rosenberg J, Jacobsen B, Fischer A. Fast-track giant paraoesophageal hernia repair using a simplified laparoscopic technique. Langenbecks Arch Surg 2006;391:38-42. [Crossref] [PubMed]
- Yates RB, Hinojosa MW, Wright AS, et al. Laparoscopic gastropexy relieves symptoms of obstructed gastric volvulus in highoperative risk patients. Am J Surg 2015;209:875-80; discussion 880. [Crossref] [PubMed]
- Bruenderman EH, Martin RCG, Kehdy FJ. Outcomes after Laparoscopic Gastropexy as an Alternative for Paraesophageal Hernia Repair. JSLS 2020;24:e2020. [Crossref] [PubMed]
- Shehzad K, Askari A, Slesser AAP, et al. A Safe and Effective Technique of Paraesophageal Hernia Reduction Using Combined Laparoscopy and Nonsutured PEG Gastropexy in High-Risk Patients. JSLS 2019;23:e2019. [Crossref] [PubMed]
- Xenos ES. Percutaneous endoscopic gastrostomy in a patient with a large hiatal hernia using laparoscopy. JSLS 2000;4:231-3. [PubMed]
- Kercher KW, Matthews BD, Ponsky JL, et al. Minimally invasive management of paraesophageal herniation in the high-risk surgical patient. Am J Surg 2001;182:510-4. [Crossref] [PubMed]
- Arevalo G, Wilkerson J, Saxe J. Acute Paraesophageal Hernia: Laparoscopic Repair With Adjunct T-Fastener Gastropexy for the High Operative Risk Patient. Surg Laparosc Endosc Percutan Tech 2018;28:123-7. [Crossref] [PubMed]
- Ponsky J, Rosen M, Fanning A, et al. Anterior gastropexy may reduce the recurrence rate after laparoscopic paraesophageal hernia repair. Surg Endosc 2003;17:1036-41. [Crossref] [PubMed]
- Diaz S, Brunt LM, Klingensmith ME, et al. Laparoscopic paraesophageal hernia repair, a challenging operation: medium-term outcome of 116 patients. J Gastrointest Surg 2003;7:59-67. [Crossref] [PubMed]
- Poncet G, Robert M, Roman S, et al. Laparoscopic repair of large hiatal hernia without prosthetic reinforcement: late results and relevance of anterior gastropexy. J Gastrointest Surg 2010;14:1910-6. [Crossref] [PubMed]
- Oor JE, Roks DJ, Broeders JA, et al. Seventeen-year Outcome of a Randomized Clinical Trial Comparing Laparoscopic and Conventional Nissen Fundoplication: A Plea for Patient Counseling and Clarification. Ann Surg 2017;266:23-8. [Crossref] [PubMed]
- Nason KS, Luketich JD, Qureshi I, et al. Laparoscopic repair of giant paraesophageal hernia results in long-term patient satisfaction and a durable repair. J Gastrointest Surg 2008;12:2066-75; discussion 2075-7. [Crossref] [PubMed]
- Chan EG, Sarkaria IS, Luketich JD, et al. Laparoscopic Approach to Paraesophageal Hernia Repair. Thorac Surg Clin 2019;29:395-403. [Crossref] [PubMed]
- Karush J, Sarkaria IS. Robotic-Assisted Giant Paraesophageal Hernia Repair and Nissen Fundoplication. Operative Techniques in Thoracic and Cardiovascular Surgery 2013;18:204-214. [Crossref]
- Rakić S, Pesko P, Dunjić MS, et al. Paraoesophageal hernia repair with and without concomitant fundoplication. Br J Surg 1994;81:1162-3. [Crossref] [PubMed]
- Palanivelu C, Rangarajan M, Shetty AR, et al. Laparoscopic suture gastropexy for gastric volvulus: a report of 14 cases. Surg Endosc 2007;21:863-6. [Crossref] [PubMed]
- Daigle CR, Funch-Jensen P, Calatayud D, et al. Laparoscopic repair of paraesophageal hernia with anterior gastropexy: a multicenter study. Surg Endosc 2015;29:1856-61. [Crossref] [PubMed]
Cite this article as: Levesque RL, Alicuben ET, Ekeke C, Luketich JD, Sarkaria IS. Use of gastropexy for paraesophageal hernias—a narrative review. Video-assist Thorac Surg 2022;7:13.