
Endosonography in mediastinal staging of lung cancer: a concise literature review
Background and rationale
Lung cancer staging is of key importance for therapeutic decision-making and for its prognostic implications. In particular, the assessment of the status of intrathoracic lymph nodes is a crucial step in patients potentially eligible for surgery. In the absence of distant metastases, surgery represents the first line treatment of non-small cell lung cancer (NSCLC) provided that the mediastinal lymph nodes are normal or reactive (1,2). On the contrary, malignant involvement of the mediastinal lymph nodes (N2 or N3 disease) defines a “locally advanced” tumor (stage III), often excludes surgery as a first line therapeutic approach, and requires a complex, multimodality treatment strategy. In particular, stage IIIA disease is usually differentiated in resectable or unresectable at the time of diagnosis. In resectable stage IIIA NSCLC (T3–4/N1, T4/N0 or T1–3 non bulky single-station N2), the standard of care should include consideration of surgical resection after multidisciplinary approach involving neoadjuvant or adjuvant chemotherapy, radiation, or both (3,4). Stage IIIB (T1–2/N3, or T3T1–2/N34/N2) and IIIC (T3T1–2/N34 N3) involve lymph node metastasis in the contralateral hemithorax or supraclavicular fossa and/or an unresectable primary tumor, making patients with this disease not ideal candidates for surgical resection. The standard treatment option for unresectable or inoperable stage IIIA, stage IIIB and IIIC disease is concurrent chemoradiation (5-7). In 2018, the standard of care for unresectable locally advanced NSCLC changed significantly after the publication of the PACIFIC trial, which demonstrated a significant survival benefit with the addition of 1 year of durvalumab after concurrent chemoradiation, regardless of the PD-L1 status (8,9).
Given the implications discussed above for the therapeutic decision-making, the mediastinal staging of lung cancer represents a key moment in the journey of a patient lacking distant metastasis at onset. While computed tomography (CT) and positron emission tomography (PET) are the first line tests in the imaging assessment of patients with suspected NSCLC, their ability to correctly define the mediastinal status is unsatisfactory owing to their relatively high rate of false negatives and false positives, which may lead to suboptimal treatment or to unnecessary surgery, respectively (1,2,10,11). In the last two decades, endosonography (endobronchial ultrasound-guided transbronchial needle aspiration, EBUS-TBNA; endoscopic ultrasound with fine needle aspiration, EUS/EUS-B-FNA), has become the first line test for mediastinal staging based on the mounting evidence about its effectiveness and safety (1,2,10-12).
Equipment and reach
The linear EBUS scope is a flexible bronchoscope with a convex ultrasound probe at its tip that enables visualization and sampling of lymph nodes (and in general of any lesion) adjacent to the airway under real-time ultrasound guidance. As for the intrathoracic lymph nodes, most mediastinal, hilar and interlobar nodes are accessible with a linear EBUS scope through the airways. The EBUS scope can be also introduced in the esogaphus and the stomach, just like a regular echogastroscope, and allow for the assessment and sampling (EUS-B-FNA) of lymph node stations which can be reached only from the esophagus (i.e., stations 8, 9) or are easier to reach from the esophagus (i.e., station 4L). Furthermore, lymph nodes located in stations #5 and #6 can be occasionally sampled using an echogastroscope.
Indications
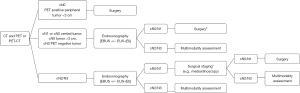
International guidelines recommend endosonography over surgical staging as the initial procedure for mediastinal staging in patients with known or suspected lung cancer associated with possible mediastinal involvement as suggested by imaging studies (lymph nodes enlarged by CT criteria and/or PET positive). A flow chart for mediastinal staging of NSCLC is shown in Figure 1.
Several studies and metanalysis confirmed the high accuracy (>90%) of endosonography for assessing the mediastinal status in patients with suspect lymph node metastases (12-14), and the ASTER trial demonstrated that EBUS is as accurate as surgery, yet less invasive, in this specific setting (15).
An invasive mediastinal assessment with endosonography is also recommended by the ACCP guidelines in patients with normal mediastinum at imaging (negative CT and PET studies) but with a central tumor and/or evidence of N1 involvement. The rationale for this approach is that radiologically-occult N2 disease can be found at surgery in up to 23.5% of these patients (1,12). The guidelines issued by the ESGE/ERS/ESTS propose that an endoscopy-based staging should be considered, even in absence of lymph node abnormalities at CT and/or PET, also in patients whose primary tumor is either ≥3 cm in long-axis size or PET negative (Figure 2) (12). However, the strength of the evidence in favour of a systematic endosonographic mediastinal staging in patients with a negative mediastinum at imaging studies is debated. First, most of the studies on which this recommendation is based enrolled a very low percentage of patients with negative mediastinum. Second, the accuracy of endosonography in this setting is widely variable. While a handful of studies demonstrated good performance characteristics of EBUS even in patients with a low prevalence of mediastinal lymph node metastases (<35%), many others achieved a low accuracy in this specific setting (16-23). In particular, a well-designed, prospective, multicenter study specifically designed to assess the prevalence of occult mediastinal metastases and the EBUS performance characteristics in patients with cN1 disease and negative mediastinum at imaging studies, confirmed the high prevalence of mediastinal involvement (24%), but showed disappointing sensitivity values (38%) for the endosonographic staging (16). Mediastinoscopy performed better (73% sensitivity) than EBUS in the same subgroup of patients, as well as in a similar group of patients enrolled by Decaluwé et al. in a subsequent prospective trial (24). However, endosonography in these group of patients was carried out only with EBUS, and most of the occult N2 metastases detected at surgical lymph node dissection could have been detected with a combined EBUS/EUS-B staging procedure (25).
Two systematic reviews with metanalysis were recently published with the aim to summarize the evidence regarding the value of endosonographic staging in patients with cN0/N1 lung cancer. Interestingly, the prevalence in the two studies ranged from 13% to 15%, the sensitivity of EBUS was identical and quite disappointing (49%), but the negative predictive value ranged from 91% to 93% (22,23).
Given the above unsatisfactory accuracy values and the publication of new evidence on the prevalence of unforeseen N2 disease in patients with cN0/N1 lung cancer, it is possible that the recommendations regarding the endosonographic staging in this setting will be updated in the near future. Furthermore, some studies published after the currently followed cancer guidelines were issued suggest that the prevalence of occult N2 disease in patients with negative mediastinum and central primary tumor or primary tumor >3 cm may be as high as 8%, thus considerably lower than previously thought. Such prevalence, in turn, might not warrant an invasive mediastinal staging in the absence of additional risk factors (i.e., N1 involvement) (26-28).
Endosonography versus mediastinoscopy
A limited number of individual studies compared the performance characteristics and the complications of endosonography versus mediastinoscopy in the mediastinal staging of lung cancer. Furthermore, three meta-analyses summarized these studies and found a comparable diagnostic value with a lower complication rate for endosonography as compared to mediastinoscopy (29-31).
Ge et al. analysed 17 studies and almost 1,000 patients and found an equivalent sensitivity for the detection of mediastinal metastases (0.84 for EBUS-TBNA versus 0.86 for mediastinoscopy). Mediastinoscopy was associated with more complication (17 vs. 4) and fewer false negatives as compared to EBUS-TBNA. Indirect meta-regressive method was used in this study to compare the two staging modalities, since no strong direct comparison data were available (29). On the contrary, Sehgal et al. provide a true head to head comparison between the two techniques. The pooled risk-difference of the sensitivity of endosonography versus mediastinoscopy was 0.11 and 0.11 respectively, suggesting equivalent performance characteristics of the two procedures. A higher major complications rate was related to mediastinoscopy (35 of 445 versus 5 of 459) including bleeding, esophageal perforation, tracheal injury, prolonged need for ventilation. However, the Authors reported a significant heterogeneity in patient population and procedures (30). In the third metanalysis, by Rossi Figueiredo et al., only 5 studies with a certain degree of heterogeneity were included. The strength of this systematic review is the broad search for prospective studies of satisfactory quality with a low risk of bias. Also in this case EBUS-TBNA and mediastinoscopy showed a similar performance for mediastinal staging of NSCLC (31).
It is important to underline that most of the studies included in the above mentioned metanalyses used either EUS-FNA or EBUS-TBNA to sample the mediastinal lymph nodes. It is likely that combining the two procedures might maximize the diagnostic accuracy of the endosonographic staging. In a randomized controlled multicenter trial comparing either surgical staging or endosonography (combined EBUS-TBNA and EUS-FNA) followed by surgical staging for mediastinal nodal staging, the surgical staging showed a sensitivity of 79% versus 85% for endosonography alone; the complication rate was respectively 6% and 1% (15). Similar results were obtained by several prospective controlled trials that directly compared the two modalities (15,32-36). Most of these studies underline that endosonography might improve the accuracy of the mediastinal staging by allowing the biopsy of lymph node stations which are not reachable by mediastinoscopy, especially if a combined EBUS/EUS approach is used (32-34).
Endosonography: staging strategy
The endosonographic mediastinal staging of lung cancer should keep carefully into account the clinical TNM stage and the intent of the procedure of each specific patient. Based on this information, the operator can implement a strategy that encompasses the use of EBUS alone versus a combination of EBUS and EUS-B, as well as the performance of a selective versus systematic staging.
In patients with clinical stage IV disease, endosonography is usually performed when the mediastinal lymph nodes are easier to reach than the primary tumor or the other metastatic sites, and the aim of the procedure is both to obtain a histologic diagnosis and to retrieve material of sufficient quality and quantity for thorough molecular profiling (Figure 3). In this setting, carrying out either an EBUS or an EUS procedure alone (depending on the location of the suspicious lymph nodes) and performing a selective sampling of one or a few lymph nodes which are likely to be metastatic based on the results of the imaging studies is justified.
In potentially operable patients lacking distant metastases at imaging studies, the thoroughness of the endosonographic staging is the key to the correct choice of the treatment. In this specific setting, evidence from the literature suggests that a systematic lymph node assessment carried out with a combined EBUS/EUS procedure is the ideal best strategy. A systematic mediastinal assessment implies that all the lymph node stations are explored and that at least the largest node with short axis size above 5 mm within each of the stations #4R, #4L and #7 are sampled even if it does not show B-mode features suggestive of malignant involvement. All the other “abnormal” intrathoracic lymph nodes, as identified by size, FDG avidity and EBUS B-mode features can be sampled if their status is considered key to establish the treatment strategy (2,12).
In a well-known randomized trial comparing EBUS-centered versus EUS-centered staging, Kang et al. demonstrated that a combined, systematic EBUS/EUS mediastinal approach is associated with the best sensitivity values, but also that a systematic EBUS staging alone is superior to a systematic EUS staging alone (82.4% vs. 60% sensitivity, respectively) (37). More recently, the SCORE study compared the outcomes of a systematic, combined EBUS/EUS staging (investigation of FDG-PET-CT suspect lymph nodes and routine sampling of stations #4R, #4L and #7 in the presence of nodes with a short axis of ≥8 mm) with a targeted EBUS staging (assessment and sampling of CT-enlarged and/or FDG-PET positive nodes only). The combined, systematic approach was found to have 9% higher sensitivity for the detection of mediastinal lymph node metastases, while additional clinically relevant staging information was found in 10% of patients (38). Finally, a meta-analysis showed a significant increase in sensitivity (+12%) and detection rate of lymph node metastases using a combined EBUS and EUS compared with either procedure alone (39).
While no study has evaluated the added value of a combined EBUS/EUS procedure versus either procedure alone in patients with cN0/N1 lung cancer, it is likely that the usefulness of a combined procedure would be even more important in this specific setting. One might in fact speculate that a thorough systematic lymph node assessment carried out with a combined EBUS/EUS-B approach might help reduce the impact of some of the factors that contribute to undermine the efficacy of the endosonography staging in patients with radiologically-occult disease. Among these, the most important are the involvement of stations which are usually out of reach (#5, #6), the presence of micrometasis, the presence of multiple lymph nodes in the same station (which makes it difficult to sample them all), and an insufficient thoroughness of the staging (i.e., lack of systematic lymph node assessment and/or sampling).
Surgery after a negative endosonography
As a false negative result is considered the main limit of endosonography, a negative EBUS and/or EUS staging should ideally be followed by a surgical evaluation if the pre-test probability of lymph node malignant involvement is thought to be high, typically in patients with nodes which are enlarged at CT or PET positive (1,12,40). However, the scientific evidence backing such an approach is scant and debated.
A recent systematic review with metanalysis involving 3,248 patients evaluated the rate of unforeseen N2 disease in patients who were submitted to lung tumor resection after a mediastinal staging carried out with endosonography alone or with endosonography followed by mediastinoscopy in case of negative endosonography. Interestingly, the rate of unforeseen N2 disease was similar (9.9% after endosonography versus 9.6% after endosonography + mediastinoscopy), at the cost of 6.0% rate of complications by mediastinoscopy (41). A large multicenter, parallel, randomized non-inferiority trial is currently ongoing with the aim of comparing a mediastinal staging performed with EBUS/EUS with or without confirmatory mediastinoscopy in 360 patients with potentially operable NSCLC (42).
Furthermore, differences in term of survival in patients undergoing lung resection after a mediastinal staging based on endosonography alone versus endosonography followed by mediastinoscopy in case of negative endosonography results have not been demonstrated, to our knowledge. Kuijvenhoven et al. reported the 5-year survival of patients enrolled in the prospective, multicenter ASTER trial, which was designed to compare the value of an endosonographic versus a surgical mediastinal staging. Interestingly, the survival was 35% in both groups (43). In an attempt to explain such an outcome, one should consider that most of the unforeseen N2 cases missed by endosonography are single station (81% in a Dutch registry), are caused by micro-metastatic lymph node involvement, and/or involve stations #5 and #6 (28). Studies demonstrate, in fact, that the worst survival rates are seen in patients with multiple station N2 disease and/or with macroscopic (>2 mm) N2 disease (28,44,45). There is also evidence suggesting that single station lymph node metastasis in the #5 or #6 locations are associated with a better 3-year survival than the involvement of other mediastinal lymph nodes in patients with a left upper lobe NSCLC (28,46).
Risk stratification
Theoretically, an accurate risk stratification of metastatic involvement of mediastinal lymph nodes would allow for a more reliable selection of patients who really need a surgical staging when endosonography does not show evidence of N2–3 disease. In particular, an optimal pre-test malignancy assessment could help identify both false positives of CT and PET and false negatives of endosonography, thus reducing the number of patients who require a confirmatory mediastinoscopy. In clinical practice, tumor histology, CT, PET, ultrasound and elastographic characteristics are the main parameters which can be taken into account to estimate a pre-test probability of malignancy for the intrathoracic lymph nodes. Several attempts have been made to use the results of these tests, alone or in various combinations, to predict the risk of malignancy of mediastinal lymph nodes, but none of them has proved reliable enough up to now.
The endosonography B-mode findings, in particular, have largely been used to try to predict the risk of malignancy of a given lymph node. Fujiwara et al. found that some B-mode features such as round shape, distinct margins, heterogeneous echogenicity, and the presence of the so-called central necrosis are independent predictors of malignancy (47). However, several subsequent studies have failed to replicate the above results and have shown significant discrepancy and subjectivity in the use of these ultrasound features, with consequent limited diagnostic utility (48-52).
Elastography is another ultrasound-based method that estimates the elasticity of a tissue and can help predict the probability of malignancy. Several pilot studies suggest that strain elastography (SE), used along with EBUS (EBUS-SE, Figure 4) can be used to differentiate between malignant and benign mediastinal lymph nodes with high accuracy and can be useful both in guiding mediastinal lymph node sampling and in stratifying the risk of malignancy in a negative sample obtained during endosonography (53-60).
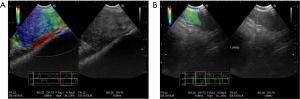
The combined assessment of EBUS-SE and EBUS B-mode findings (61), as well as the combined use of the information retrieved with CT, PET and EBUS-SE have shown promising results in their ability to correctly predict the metastatic intrathoracic lymph node involvement (60).
Finally, Ceron et al. have designed a mathematical model that tries to predict the probability of nodal metastasis after CT, PET and endosonography results by using the Bayes’ theorem (62). The model is currently being tested in a prospective multicenter study.
Conclusions
Endosonography has revolutionized the invasive mediastinal staging of lung cancer and is certainly the best first choice in potentially operable patients with either enlarged (>1 cm on the short axis) or PET positive mediastinal lymph nodes. The sensitivity and accuracy of endosonography in the mediastinal staging of cN0/N1 patients seem significantly worse, but they have only been assessed in a handful of studies, often of poor quality. Furthermore, it is currently unclear if performing a surgical mediastinal staging in a patient with a negative endosonography assessment is associated with any advantage in terms of survival after lung resection.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Chiappetta and Francesco Facciolo) for the series “Lymphadenectomy during VATS and RATS: state of the art” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-21-25/coif). The series “Lymphadenectomy during VATS and RATS: state of the art” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Silvestri GA, Gonzales AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-e250S.
- De Leyn P, Dooms C, Kuzdzal J, et al. Preoperative mediastinal lymph node staging for non-small cell lung cancer: 2014 update of the 2007 ESTS guidelines. Transl Lung Cancer Res 2014;3:225-33. [PubMed]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442-50. [Crossref] [PubMed]
- Eberhardt WE, Pöttgen C, Gauler TC, et al. Phase III Study of Surgery Versus Definitive Concurrent Chemoradiotherapy Boost in Patients With Resectable Stage IIIA(N2) and Selected IIIB Non-Small-Cell Lung Cancer After Induction Chemotherapy and Concurrent Chemoradiotherapy (ESPATUE). J Clin Oncol 2015;33:4194-201. [Crossref] [PubMed]
- Rowell NP, O'rourke NP. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2004;CD002140. [PubMed]
- O'Rourke N, Roqué I. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2010;CD002140. [Crossref] [PubMed]
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw 2018;16:807-21. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and oesophageal endosonography for the diagnosis and staging of lung cancer. European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Eur Respir J 2015;46:40-60. [Crossref] [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [Crossref] [PubMed]
- Dong X, Qiu X, Liu Q, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the mediastinal staging of non-small cell lung cancer: a meta-analysis. Ann Thorac Surg 2013;96:1502-7. [Crossref] [PubMed]
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [Crossref] [PubMed]
- Dooms C, Tournoy KG, Schuurbiers O, et al. Endosonography for mediastinal nodal staging of clinical N1 non-small cell lung cancer: a prospective multicenter study. Chest 2015;147:209-15. [Crossref] [PubMed]
- Herth FJ, Ernst A, Eberhardt R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically normal mediastinum. Eur Respir J 2006;28:910-4. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Krasnik M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest 2008;133:887-91. [Crossref] [PubMed]
- Szlubowski A, Zieliński M, Soja J, et al. A combined approach of endobronchial and endoscopic ultrasound-guided needle aspiration in the radiologically normal mediastinum in non-small-cell lung cancer staging--a prospective trial. Eur J Cardiothorac Surg 2010;37:1175-79. [Crossref] [PubMed]
- Ong P, Grosu H, Eapen GA, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for systematic nodal staging of lung cancer in patients with N0 disease by computed tomography and integrated positron emission tomography-computed tomography. Ann Am Thorac Soc 2015;12:415-9. [Crossref] [PubMed]
- Shingyoji M, Nakajima T, Yoshino M, et al. Endobronchial ultrasonography for positron emission tomography and computed tomography-negative lymph node staging in non-small cell lung cancer. Ann Thorac Surg 2014;98:1762-7. [Crossref] [PubMed]
- El-Osta H, Jani P, Mansour A, et al. Endobronchial Ultrasound for Nodal Staging of Patients with Non-Small-Cell Lung Cancer with Radiologically Normal Mediastinum. A Meta-Analysis. Ann Am Thorac Soc 2018;15:864-74. [Crossref] [PubMed]
- Leong TL, Loveland PM, Gorelik A, et al. Preoperative Staging by EBUS in cN0/N1 Lung Cancer: Systematic Review and Meta-Analysis. J Bronchology Interv Pulmonol 2019;26:155-65. [Crossref] [PubMed]
- Decaluwé H, Dooms C, D'Journo XB, et al. Mediastinal staging by videomediastinoscopy in clinical N1 non-small cell lung cancer: a prospective multicentre study. Eur Respir J 2017;50:1701493. [Crossref] [PubMed]
- Annema JT. Endosonographic staging for N1 disease. Chest 2015;147:e122. [Crossref] [PubMed]
- Decaluwé H, Moons J, Fieuws S, et al. Is central lung tumour location really predictive for occult mediastinal nodal disease in (suspected) non-small-cell lung cancer staged cN0 on 18F-fluorodeoxyglucose positron emission tomography-computed tomography? Eur J Cardiothorac Surg 2018;54:134-40. [Crossref] [PubMed]
- Casal RF, Sepesi B, Sagar AS, et al. Centrally located lung cancer and risk of occult nodal disease: an objective evaluation of multiple definitions of tumour centrality with dedicated imaging software. Eur Respir J 2019;53:1802220. [Crossref] [PubMed]
- Bousema JE, Heineman DJ, Dijkgraaf MGW, et al. Adherence to the mediastinal staging guideline and unforeseen N2 disease in patients with resectable non-small cell lung cancer: Nationwide results from the Dutch Lung Cancer Audit - Surgery. Lung Cancer 2020;142:51-8. [Crossref] [PubMed]
- Ge X, Guan W, Han F, et al. Comparison of Endobronchial Ultrasound-Guided Fine Needle Aspiration and Video-Assisted Mediastinoscopy for Mediastinal Staging of Lung Cancer. Lung 2015;193:757-66. [Crossref] [PubMed]
- Sehgal IS, Dhooria S, Aggarwal AN, et al. Endosonography Versus Mediastinoscopy in Mediastinal Staging of Lung Cancer: Systematic Review and Meta-Analysis. Ann Thorac Surg 2016;102:1747-55. [Crossref] [PubMed]
- Figueiredo VR, Cardoso PFG, Jacomelli M, et al. EBUS-TBNA versus surgical mediastinoscopy for mediastinal lymph node staging in potentially operable non-small cell lung cancer: a systematic review and meta-analysis. J Bras Pneumol 2020;46:e20190221. [Crossref] [PubMed]
- Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008;3:577-82. [Crossref] [PubMed]
- Liberman M, Sampalis J, Duranceau A, et al. Endosonographic mediastinal lymph node staging of lung cancer. Chest 2014;146:389-97. [Crossref] [PubMed]
- Um SW, Kim HK, Jung SH, et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J Thorac Oncol 2015;10:331-7. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]
- Czarnecka-Kujawa K, Yasufuku K. The role of endobronchial ultrasound versus mediastinoscopy for non-small cell lung cancer. J Thorac Dis 2017;9:S83-97. [Crossref] [PubMed]
- Kang HJ, Hwangbo B, Lee GK, et al. EBUS-centred versus EUS-centred mediastinal staging in lung cancer: a randomised controlled trial. Thorax 2014;69:261-8. [Crossref] [PubMed]
- Crombag LMM, Dooms C, Stigt JA, et al. Systematic and combined endosonographic staging of lung cancer (SCORE study). Eur Respir J 2019;53:1800800. [Crossref] [PubMed]
- Korevaar DA, Crombag LM, Cohen JF, et al. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: a systematic review and meta-analysis. Lancet Respir Med 2016;4:960-8. [Crossref] [PubMed]
- Tournoy K, Rintoul R, Dooms C, et al. Can mediastinoscopy after negative endosonography in lung cancer be omitted? Subanalysis of ASTER with focus on PET. Eur Respir J 2011;38:1973.
- Bousema JE, van Dorp M, Noyez VJJM, et al. Unforeseen N2 Disease after Negative Endosonography Findings with or without Confirmatory Mediastinoscopy in Resectable Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. J Thorac Oncol 2019;14:979-92. [Crossref] [PubMed]
- Bousema JE, Dijkgraaf MGW, Papen-Botterhuis NE, et al. MEDIASTinal staging of non-small cell lung cancer by endobronchial and endoscopic ultrasonography with or without additional surgical mediastinoscopy (MEDIASTrial): study protocol of a multicenter randomised controlled trial. BMC Surg 2018;18:27. [Crossref] [PubMed]
- Kuijvenhoven JC, Korevaar DA, Tournoy KG, et al. Five-Year Survival After Endosonography vs Mediastinoscopy for Mediastinal Nodal Staging of Lung Cancer. JAMA 2016;316:1110-2. [Crossref] [PubMed]
- Yoo C, Yoon S, Lee DH, et al. Prognostic Significance of the Number of Metastatic pN2 Lymph Nodes in Stage IIIA-N2 Non-Small-Cell Lung Cancer After Curative Resection. Clin Lung Cancer 2015;16:e203-12. [Crossref] [PubMed]
- Garelli E, Renaud S, Falcoz PE, et al. Microscopic N2 disease exhibits a better prognosis in resected non-small-cell lung cancer. Eur J Cardiothorac Surg 2016;50:322-8. [Crossref] [PubMed]
- Citak N, Sayar A, Metin M, et al. The Prognostic Significance of Metastasis to Lymph Nodes in Aortopulmonary Zone (Stations 5 and 6) in Completely Resected Left Upper Lobe Tumors. Thorac Cardiovasc Surg 2015;63:568-76. [Crossref] [PubMed]
- Fujiwara T, Yasufuku K, Nakajima T, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest 2010;138:641-7. [Crossref] [PubMed]
- Evison M, Morris J, Martin J, et al. Nodal staging in lung cancer: a risk stratification model for lymph nodes classified as negative by EBUS-TBNA. J Thorac Oncol 2015;10:126-33. [Crossref] [PubMed]
- Dietrich CF, Jenssen C, Herth FJ. Endobronchial ultrasound elastography. Endosc Ultrasound 2016;5:233-8. [Crossref] [PubMed]
- Wahidi MM, Herth F, Yasufuku K, et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Satterwhite LG, Berkowitz DM, Parks CS, et al. Central intranodal vessels to predict cytology during endobronchial ultrasound transbronchial needle aspiration. J Bronchology Interv Pulmonol 2011;18:322-8. [Crossref] [PubMed]
- Wang Memoli JS, El-Bayoumi E, Pastis NJ, et al. Using endobronchial ultrasound features to predict lymph node metastasis in patients with lung cancer. Chest 2011;140:1550-6. [Crossref] [PubMed]
- Shiina T, Nightingale KR, Palmeri ML, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol 2015;41:1126-47. [Crossref] [PubMed]
- Itoh A, Ueno E, Tohno E, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology 2006;239:341-50. [Crossref] [PubMed]
- Izumo T, Sasada S, Chavez C, et al. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol 2014;44:956-62. [Crossref] [PubMed]
- Huang H, Huang Z, Wang Q, et al. Effectiveness of the Benign and Malignant Diagnosis of Mediastinal and Hilar Lymph Nodes by Endobronchial Ultrasound Elastography. J Cancer 2017;8:1843-8. [Crossref] [PubMed]
- Dietrich CF, Jenssen C, Arcidiacono PG, et al. Endoscopic ultrasound: Elastographic lymph node evaluation. Endosc Ultrasound 2015;4:176-90. [Crossref] [PubMed]
- Xu W, Shi J, Zeng X, et al. EUS elastography for the differentiation of benign and malignant lymph nodes: a meta-analysis. Gastrointest Endosc 2011;74:1001-9; quiz 1115.e1-4. [Crossref] [PubMed]
- Dietrich CF, Săftoiu A, Jenssen C. Real time elastography endoscopic ultrasound (RTE-EUS), a comprehensive review. Eur J Radiol 2014;83:405-14. [Crossref] [PubMed]
- Verhoeven RLJ, Trisolini R, Leoncini F, et al. Predictive Value of Endobronchial Ultrasound Strain Elastography in Mediastinal Lymph Node Staging: The E-Predict Multicenter Study Results. Respiration 2020;99:484-92. [Crossref] [PubMed]
- Fujiwara T, Nakajima T, Inage T, et al. The combination of endobronchial elastography and sonographic findings during endobronchial ultrasound-guided transbronchial needle aspiration for predicting nodal metastasis. Thorac Cancer 2019;10:2000-5. [Crossref] [PubMed]
- Ceron L, Michieletto L, Zamperlin A. Mediastinal staging in lung cancer: a rational approach. Monaldi Arch Chest Dis 2009;71:170-5. [PubMed]
Cite this article as: Leoncini F, Magnini D, Livi V, Flore MC, Porro LM, Paioli D, Trisolini R. Endosonography in mediastinal staging of lung cancer: a concise literature review. Video-assist Thorac Surg 2022;7:4.


