
A narrative review of paraconduit hernia after esophagectomy: where are we now?
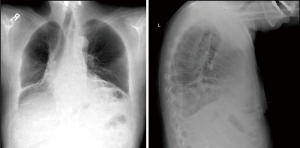
Esophagectomy is the standard of care for the treatment of esophageal cancers in appropriate surgical candidates. The procedure involves resection of the esophagus and tumor with creation of a new alimentary conduit, most often via gastric interposition with esophagogastric anastomosis either in the neck or chest (1). Paraesophageal hernia is a known complication of esophagectomy with estimates of incidence typically ranging from 0.4% to 15% depending on inclusion criteria (2-8). The majority of paraconduit hernias are often asymptomatic and only detected via radiographic imaging, which in itself can present unique challenges given the complex post-surgical anatomy (Figure 1, Video 1) (3,6). Nevertheless, complications are potentially life-threatening including respiratory distress, incarceration, and perforation. Indications and timing for repair remains disputed, with a paucity of data detailing operative techniques for repair as well as prevention of paraconduit hernias.
Minimally invasive esophagectomy (MIE) has emerged as an oncologic equivalent to open abdominal or transthoracic approaches, and generally considered to be a relatively less morbid strategy. Yet, somewhat paradoxically, the rate of paraconduit hernias has risen commensurate with the popularity of minimally invasive approaches (6,7,9-11). Moreover, there exist many variations to MIE, including totally minimally invasive vs. hybrid techniques, as well as different surgical approaches, including transhiatal without thoracic access and transthoracic mobilization with intrathoracic (Ivor Lewis) or cervical esophageal anastomosis (McKeown), that further confound identification of associated risk factors (3,9,12).
In this review, we focus on the most recent literature detailing the rise in incidence of paraconduit hernias with MIE techniques, comparing different surgical approaches and identifying potential risk factors. We also briefly discuss indications and techniques for repair of paraesophageal hernias as well as suggestions for hernia prevention.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://vats.amegroups.com/article/view/10.21037/vats-21-28/rc).
Open vs. MIE
We performed a literature review using the PubMed database including studies describing the incidence rate, operative techniques, and diagnosis of paraconduit hernias, with a specific focus on recent advances over the past decade to provide a current assessment of the management for this post-esophagectomy complication.
The introduction of MIE has yielded fewer wound infections, lower post-operative pain, shorter duration of hospital stay, and reduced in-hospital mortality rate, while maintaining equivalent oncologic outcomes compared to open esophagectomy. In contrast, MIE is associated with a significantly higher incidence of post-esophagectomy hernia (6,7,9-11). A recent meta-analysis that included twenty-six studies between 1985 to 2015 with a total of 6,058 patients (n=3,621 open, n=2,437 MIE), found that 240 patients were eventually diagnosed with hiatal hernia during long-term follow up (9). The pooled incidence was determined to be 4.5% (95% CI: 2.8–6.2) vs. 1.0% (95% CI: 0.6–1.3) after MIE and open esophagectomy, respectively. Of note, the median time interval to diagnosis of hiatal hernia was considerably shorter for MIE, 8.8 months (range, 6–29 months), compared to open esophagectomy, 21 months (range, 9–31 months).
Patients are likely predisposed to hernia following esophagectomy given the trans-diaphragmatic pressure gradient created by positive intra-abdominal pressure and negative intra-thoracic pressure across the widened hiatus. Various mechanisms have been suggested for how minimally invasive techniques could enhance this effect. Fewer peritoneal adhesions following MIE compared to open procedures is often cited as one reason for the increased incidence of hiatal hernia (3,6,7). Peritoneal adhesions are thought to anchor the abdominal viscera and secure the hiatus, wherein their absence may allow abdominal contents to traverse the hiatus more freely. This hypothesis would be consistent with the notably shorter time to occurrence. Additionally, iatrogenic hiatal widening may be more extensive during MIE given both the instrumentation and the gas insufflation necessary to adequately perform mediastinal dissection and mobilization of the esophagus (4,5). It has been suggested that the greater visualization afforded by minimally invasive techniques, including robot-assisted approaches, may inadvertently cause further attenuation of the crura sustained during prolonged retraction with instrumentation unique to this approach (11).
Surgical approach
Currently, the most common surgical techniques for esophagectomy, whether open or minimally invasive, include transhiatal, Ivor Lewis, and McKeown approaches. MIE may be performed either totally minimally invasively or via hybrid techniques including hand-assisted laparoscopic surgery and more recently with robot assistance (4,9,11-13). Given the variety of surgical approaches, there are an insufficient number of studies to perform an in-depth comparison of esophagectomy techniques; however, reports from individual institutional experience provide some insight.
In one of the largest single-center studies, Price et al. found no difference in hernia incidence when comparing Ivor Lewis (9 of 978; 0.92%) and transhiatal (5 of 601; 0.83%) approaches overall (5). With respect to open techniques, Messenger et al. found that patients who developed diaphragmatic hernias in their open cohort had undergone transhiatal esophagectomy (2 of 205; 1.0%) (13). This is consistent with Ganeshan et al. who reported the highest incidence of post-esophagectomy diaphragmatic hernia in open transhiatal esophagectomy (21 of 105; 20%), likely due to the need for extended hiatal enlargement, which they argued plays the major role in hernia incidence (3). Somewhat in contrast to prior reports, however, they found comparable rates of occurrence among open Ivor Lewis (18 of 267; 6.7%) and McKeown (3 or 38; 7.9%) and MIE (2 of 30; 6.6%) when fat-only hernias were excluded from the analysis.
For minimally invasive techniques, Gooszen et al. observed the highest incidence after minimally invasive Ivor Lewis esophagectomy (9.4%) as compared with all other procedures (8). Another study similarly identified the majority of diaphragmatic hernias in their minimally invasive cohort had thoracic anastomoses (9 of 68; 13.2%), suggesting this approach may confer a slight susceptibility for post-esophagectomy hernia development (13).
Finally, transhiatal robot-assisted esophagectomy has gained traction as an accepted minimally invasive procedure both in our own experience and elsewhere. This approach carries a similar mortality advantage as MIE while eliminating the need for thoracotomy or thoracoscopy. Despite these benefits, Sutherland et al. reported a relatively high incidence of hiatal hernia (7 of 36; 19.4%), hypothesizing that high mediastinal dissection with the robotic arms may cause extended iatrogenic enlargement of hiatus predisposing to hernia occurrence (11).
Predictive risk factors
Neoadjuvant chemotherapy (NAC), with or without concomitant radiation, is a frequently suggested risk factor for post-operative diaphragmatic hernia given the large number of patients undergoing therapy prior to surgery and its association with delayed wound healing. Iwasaki et al. reported nearly all patients who developed hiatal hernia (10 of 11; 90.9%) following minimally invasive McKeown esophagectomy had been administered NAC, which was significantly higher than in patients without post-operative hiatal hernia (56 of 102; 54.9%) (14). Others have similarly found an increased incidence of symptomatic hernia in patients undergoing NAC, although not reaching statistical significance on multivariable analysis (3, 8). In contrast, Lung et al. found that neoadjuvant chemotherapy (22% vs. 44%, P=0.01) and radiation (31% vs. 50%, P=0.03) were protective of hernia development (6). They surmised the inflammatory and fibrosing effect to the hiatus as well as more extensive dissection following neoadjuvant radiation led to greater adhesion formation. The mechanism underlying the discrepancies in these data remains unclear given relatively similar rates of NAC between studies.
The effect of body mass index (BMI) on the development of paraconduit hernia is also debated. Ganeshan et al. found that patients with BMI >25 kg/m2 were less prone to developing post-operative hiatal hernia (3), a finding further substantiated by Lung et al. who surmised the abdominal viscera would be less mobile as a result of more intra-abdominal fat. In contrast, Benjamin et al. argued that greater adipose volume actually increased intra-abdominal pressure to explain their observation that increased BMI was actually a risk factor for post-esophagectomy diaphragmatic hernia (6,10). Given the relatively small differences between groups as well as contradictory studies, the overall effect of BMI on hernia incidence is inconclusive. Alternatively, it is possible that low preoperative BMI or significant weight loss could disproportionately reduce the amount of perigastric adipose tissue, in turn, predisposing to paraconduit herniation, although few data exist.
Pre-existing diagnosis of hiatal hernia may also predict development of incarcerated hernia post-operatively. In a recent case series, nearly all patients who developed hiatal hernia following robot-assisted esophagectomy had a pre-existing diagnosis of the same (6 of 7; 85.7%), whereas patients without post-operative incarcerations were significantly less likely to have had prior hiatal hernia (11 of 29; 37.9%) (11). Additionally, intra-operative challenges such as large, bulky tumors of the esophagogastric junction requiring diaphragmatic resection, or the need to enter the left pleural cavity may similarly increase the risk for post-esophagectomy hernia.
Diagnosis
Post-esophagectomy hernia occurs most frequently in the left chest, either due to adhesions between the conduit and liver or the space-occupying effect of the gastric conduit as it is typically positioned in the right-side of the chest (4). CT scan remains the preferred imaging modality for patients with suspected paraconduit hernia (9). Given the complex post-surgical anatomy, however, it can be challenging to distinguish hernia contents from the normal components of the esophageal reconstruction. In a recent study, patients underwent routine CT surveillance every 3 months for 2 years, then every 6 months until five years, and annually thereafter (6). Of the 36 paraconduit hernias detected radiographically, only 21 (58%) were initially identified by the original radiology report. Ganeshan et al. previously argued this condition may be even more underrecognized, reporting that only 7 out of 67 (10%) post-esophagectomy diaphragmatic hernias were prospectively identified by the reviewing radiologists (3). These findings highlight the importance of increased awareness and communication between the surgery team and radiologists when reviewing post-operative diagnostic imaging.
Repair of paraconduit hernias
It is generally accepted that patients with symptomatic paraconduit hernias should undergo repair given they are suitably fit for surgery (9). In one of the larger studies to date, Kent et al., found that 2.8% and 0.8% of patients developed paraconduit hernias after MIE (N=581) and open esophagectomy (N=494), respectively leading to their recommendation for operative repair for all patients (4). In comparison, a number of studies have observed that a considerable portion of paraconduit hernias may be asymptomatic, raising the question whether or not repair is always necessary (3,6,8). This non-operative approach, however, must be weighed against the risks of rapid progression, incarceration, and subsequent perforation, which can be life-threatening especially given the relatively high frequency of urgent repairs (22%). The pooled morbidity rate after repair is approximately 25%, but can be even higher for emergent procedures with a reported 8-20% mortality (6,15,16).
In a recent study, Lung et al. was able to examine the natural history of paraconduit hernias as per the institutional routine to defer repair until a patient developed symptoms (6). Of the symptomatic patients, 11 of 13 (92%) underwent repair, 6 of which were performed emergently. This is in contrast to 3 of the 23 asymptomatic patients (13%) undergoing repair, with only one requiring emergency operation after becoming acutely symptomatic. Of note, 14 (61%) patients were found to have recurrent disease and not considered for surgical repair. Nevertheless, their experience suggests it may be reasonable to consider close surveillance in asymptomatic patients, particularly for patients with poor prognosis given the risk for treatment delays in those already attempting to recover from major surgery.
While there exist many techniques for repair of post-esophagectomy hernias, laparoscopy is considered to be the preferred approach. Benefits include initial evaluation of metastatic disease, shorter recovery time and reduced postoperative pain, while superior visualization of the right gastroepiploic artery supplying the gastric conduit makes it especially appealing for paraconduit hernia repair (17).
In cases when a tension-free closure cannot be achieved with sutures alone, mesh can be added to reinforce the hiatal repair. However, its use remains controversial given concern for erosion into the gastric conduit and its vascular supply, particularly with nonabsorbable subtypes (18). Historically, the use of prosthetic mesh repair in the general population was initially thought to confer a significant decrease in recurrence rates, although long-term follow up of these patients revealed no outcome differences (19-21). A recent randomized control trial (N=126) compared three methods of repair using sutures, absorbable mesh, and nonabsorbable mesh, finding no statistically significant difference among recurrence rates (23.1%, 30.8%, and 12.8%, respectively; P=0.161) (22,23). Similar rates of recurrence have been observed for post-esophagectomy hernia repair with and without mesh (30% vs. 27%, respectively) (4). Despite this, judicious use of mesh may be warranted when few other options exist to achieve an adequate repair. Additional techniques utilized to reinforce the hiatal repair include omentoplasty, in which the omental pedicle is lateralized and tacked down to cover the hernia defect, or mobilization of the ligamentum teres hepatis to help support and buttress the hiatus.
Options for preventive techniques
Studies directly comparing preventive techniques for post-esophagectomy hernia are lacking (9). Recommendations are mostly anecdotal, including some of those discussed here. Efforts should be taken to minimize mechanical retraction and instrumenting of the hiatus as minimally invasive approaches, particularly with robot-assisted esophagectomy, can cause exaggerated dilation of the hiatus. Operatively there is a preference for anterior vs. lateral division to prevent excessive widening of the hiatus when the crura must be divided (5), while sutures may be placed after passage of the conduit into the chest to eliminate redundancies in the aperture as needed. Finally, fixation of the conduit by tacking it to either to the crura or diaphragm has also been described (3,4,6), although long-term comparisons of fixation vs. non-fixation are not yet available. Careful attention must be paid to avoid trauma to the gastroepiploic arcade that serves as the primary vascular supply to the gastric conduit, particularly when tacking the conduit in place. Nevertheless, this approach does offer the added benefit of reducing conduit tortuosity.
Conclusions
Paraconduit hernia is a known complication following esophagectomy that has become more common with the rise of minimally invasive techniques. Incidence rates may be slightly higher with transthoracic Ivor Lewis approach for MIE, although instrumentation unique to the increasingly utilized transhiatal robot-assisted esophagectomy may also cause iatrogenic hiatus enlargement associated with similar rates of hernia occurrence. Symptomatic patients should undergo surgical repair when able with careful consideration of prosthetic mesh use, while surveillance may be reasonable in patients without symptoms, particularly for those with limited life expectancy given the associated morbidity and nontrivial hernia recurrence rates. Laparoscopic repair is especially appealing given the ability to evaluate for metastatic disease as well as superior visualization of the right gastroepiploic artery supplying the gastric conduit. Future studies should examine surgical techniques for prevention of post-esophagectomy hernia.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Rishindra M. Reddy) for the series “Paraesophageal Hiatal Hernia Repairs, Transthoracic, Transabdominal, Laparoscopic, or Robotic, Which Method is Best” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://vats.amegroups.com/article/view/10.21037/vats-21-28/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://vats.amegroups.com/article/view/10.21037/vats-21-28/coif). The series “Paraesophageal Hiatal Hernia Repairs, Transthoracic, Transabdominal, Laparoscopic, or Robotic, Which Method is Best” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Orringer MB, Marshall B, Chang AC, et al. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg 2007;246:363-72; discussion 372-4. [Crossref] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
- Ganeshan DM, Correa AM, Bhosale P, et al. Diaphragmatic hernia after esophagectomy in 440 patients with long-term follow-up. Ann Thorac Surg 2013;96:1138-45. [Crossref] [PubMed]
- Kent MS, Luketich JD, Tsai W, et al. Revisional surgery after esophagectomy: an analysis of 43 patients. Ann Thorac Surg 2008;86:975-83; discussion 967-74. [Crossref] [PubMed]
- Price TN, Allen MS, Nichols FC 3rd, et al. Hiatal hernia after esophagectomy: analysis of 2,182 esophagectomies from a single institution. Ann Thorac Surg 2011;92:2041-5. [Crossref] [PubMed]
- Lung K, Carroll PA, Rogalla P, et al. Paraconduit Hernia in the Era of Minimally Invasive Esophagectomy: Underdiagnosed? Ann Thorac Surg 2021;111:1812-9. [Crossref] [PubMed]
- Bronson NW, Luna RA, Hunter JG, et al. The incidence of hiatal hernia after minimally invasive esophagectomy. J Gastrointest Surg 2014;18:889-93. [Crossref] [PubMed]
- Gooszen JAH, Slaman AE, van Dieren S, et al. Incidence and Treatment of Symptomatic Diaphragmatic Hernia After Esophagectomy for Cancer. Ann Thorac Surg 2018;106:199-206. [Crossref] [PubMed]
- Oor JE, Wiezer MJ, Hazebroek EJ. Hiatal Hernia After Open versus Minimally Invasive Esophagectomy: A Systematic Review and Meta-analysis. Ann Surg Oncol 2016;23:2690-8. [Crossref] [PubMed]
- Benjamin G, Ashfaq A, Chang YH, et al. Diaphragmatic hernia post-minimally invasive esophagectomy: a discussion and review of literature. Hernia 2015;19:635-43. [Crossref] [PubMed]
- Sutherland J, Banerji N, Morphew J, et al. Postoperative incidence of incarcerated hiatal hernia and its prevention after robotic transhiatal esophagectomy. Surg Endosc 2011;25:1526-30. [Crossref] [PubMed]
- van Workum F, Berkelmans GH, Klarenbeek BR, et al. McKeown or Ivor Lewis totally minimally invasive esophagectomy for cancer of the esophagus and gastroesophageal junction: systematic review and meta-analysis. J Thorac Dis 2017;9:S826-33. [Crossref] [PubMed]
- Messenger DE, Higgs SM, Dwerryhouse SJ, et al. Symptomatic diaphragmatic herniation following open and minimally invasive oesophagectomy: experience from a UK specialist unit. Surg Endosc 2015;29:417-24. [Crossref] [PubMed]
- Iwasaki H, Tanaka T, Miyake S, et al. Postoperative hiatal hernia after minimally invasive esophagectomy for esophageal cancer. J Thorac Dis 2020;12:4661-9. [Crossref] [PubMed]
- Matthews J, Bhanderi S, Mitchell H, et al. Diaphragmatic herniation following esophagogastric resectional surgery: an increasing problem with minimally invasive techniques? : Post-operative diaphragmatic hernias. Surg Endosc 2016;30:5419-27. [Crossref] [PubMed]
- Andreou A, Pesthy S, Struecker B, et al. Incidence and Risk Factors of Symptomatic Hiatal Hernia Following Resection for Gastric and Esophageal Cancer. Anticancer Res 2017;37:7031-6. [PubMed]
- Erkmen CP, Raman V, Ghushe ND, et al. Laparoscopic repair of hiatal hernia after esophagectomy. J Gastrointest Surg 2013;17:1370-4. [Crossref] [PubMed]
- Ulloa Severino B, Fuks D, Christidis C, et al. Laparoscopic repair of hiatal hernia after minimally invasive esophagectomy. Surg Endosc 2016;30:1068-72. [Crossref] [PubMed]
- Frantzides CT, Madan AK, Carlson MA, et al. A prospective, randomized trial of laparoscopic polytetrafluoroethylene (PTFE) patch repair vs simple cruroplasty for large hiatal hernia. Arch Surg 2002;137:649-52. [Crossref] [PubMed]
- Oelschlager BK, Pellegrini CA, Hunter J, et al. Biologic prosthesis reduces recurrence after laparoscopic paraesophageal hernia repair: a multicenter, prospective, randomized trial. Ann Surg 2006;244:481-90. [PubMed]
- Oelschlager BK, Pellegrini CA, Hunter JG, et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg 2011;213:461-8. [Crossref] [PubMed]
- Watson DI, Thompson SK, Devitt PG, et al. Laparoscopic repair of very large hiatus hernia with sutures versus absorbable mesh versus nonabsorbable mesh: a randomized controlled trial. Ann Surg 2015;261:282-9. [Crossref] [PubMed]
- Watson DI, Thompson SK, Devitt PG, et al. Five Year Follow-up of a Randomized Controlled Trial of Laparoscopic Repair of Very Large Hiatus Hernia With Sutures Versus Absorbable Versus Nonabsorbable Mesh. Ann Surg 2020;272:241-7. [Crossref] [PubMed]
Cite this article as: Mondoñedo JR, Chang AC. A narrative review of paraconduit hernia after esophagectomy: where are we now? Video-assist Thorac Surg 2022;7:8.


