Segment-specific lymph node dissection and evaluation during anatomical pulmonary segmentectomy
Lung cancer continues to be one of the most commonly diagnosed cancer and leading cause of cancer related deaths worldwide (1). Historically only one-third of the patients with lung cancer presented with early stage disease. The growing acceptance of the low dose computed tomography (LDCT) for lung cancer screening is changing the paradigm with the expectation that two-third of all lung cancers in the screening population will be detected in their early stages (2). These patients will be potentially surgically resectable and may be candidate for segmentectomy (3). Lymph node metastasis in lung cancer is associated with poor outcomes. Overall nodal upstaging can occur up to 18% of clinical stage 1 lung cancer (4-7). Skip lymph node metastasis to N2 only nodes occurs in 4–7% of patients and N2 upstaging can be found up to 8.8% of clinical stage 1A non-small cell lung cancer (NSCLC) (4). Usual lymph node metastasis travels from intraparenchymal stations to the interlobar stations and then to hilar and mediastinal lymph nodes with worsening outcomes. The higher number of lymph node harvested and nodal upstaging are considered a surrogate for good quality oncological surgical resection. This has led to recommendation of the systemic mediastinal lymph node dissection during lung cancer surgery. The technique and method of a methodological intraoperative evaluation and removal of mediastinal lymph nodes focusing on N2 lymph node dissection is well described (8). Recently, many reports, especially from Asia, has questioned the benefit of routine aggressive mediastinal lymph node dissection (9,10). The patterns of nodal metastasis vary by the size and location of the tumor. For example, the incidence of level 7, 8 & 9 lymph node (lower mediastinal lymph nodes) metastasis is less than 1% for a <2 cm right upper lobe peripheral adenocarcinoma (9). Similarly, small peripheral lower lobe tumors preferentially metastasis to the lower mediastinal lymph nodes. This has become the basis of recommendation for lobe-specific mediastinal lymph node dissection in few centers. Hattori et al. didn’t find any survival benefit for part solid clinical stage 1 adenocarcinoma when they underwent systemic mediastinal lymph node dissection as compare to lobe-specific mediastinal lymph node dissection or hilar only lymphadenectomy (10). In contrary, the standard uptake value (SUV) of the positron emission topography (PET) and carcinoembryonic antigen (CEA) levels showed a better correlation with nodal upstaging as compare to the extent of lymphadenectomy (10).
The debate over the role of segmentectomy as an alternative to lobectomy for stage 1 lung cancer was started by Jensik et al., when they showed equivalent survival between segmentectomy and lobectomy in their series presented in American Association of Thoracic Surgeons in 1971 (11).
Two decades later, Lung Cancer Study Group (LCSG) showed significantly worse recurrence and mortality with segmentectomy as compare to lobectomy (12). Although this study had several flaws but it established lobectomy as the preferred approach for stage 1 lung cancer over segmentectomy for next decade. Subsequently, reports from Japan showed equivalent survival between segmentectomy and lobectomy for tumor less than 2 cm (13). Multiple subsequent meta-analysis also showed equivalent outcomes between segmentectomy and lobectomy for tumor less than 2 cm in size (14-17). Lutfi et al., recently reviewed the National Cancer Database to evaluate the impact of nodal upstaging in patients who underwent segmentectomy. There was no difference in the overall survival in patients with nodal upstaging who had segmentectomy or lobectomy (18).
Lobectomy is perceived as a better surgical approach by many authorities with the assumption that lobectomy allows for a more radical lymphadenectomy and better margins thus decreasing the chances of local recurrence. Although no randomized study is available yet to prove that segmentectomy and lobectomy have similar outcome for stage 1 lung cancer, but single institutional reports and meta-analyses have shown similar outcomes for at least T1a and T1b tumors.
In our experience at West Virginia University Medicine lobectomy was associated with 20% nodal upstaging as compare to 10% with segmentectomy. Though the nodal upstaging was higher in lobectomy group, the number of lymph nodes harvested in patients who had nodal upstaging was similar in both lobectomy and segmentectomy group leading to the conclusion that it is not the number of lymph node harvested but the characteristics and location of tumor that influences the nodal metastasis.
Our indications for segmentectomy are:
- Tumor less than 2 cm;
- Peripherally located;
- Preferentially ground-glass opacity or part solid;
- Confined to a segment and segmentectomy would lead to acceptable margins;
- Systemic lymph node dissection for N1 and N2 nodes with intraoperative frozen sections.
We perform intraoperative segment specific lymph node evaluation with frozen section before committing to segmentectomy. Consideration must be given to the logistic of multiple intraoperative frozen sections which can be resource consuming and may not be feasible in every institution.
In this manuscript, we are sharing the West Virginia University’s method of systemic lymph node dissection and segment specific intraoperative lymph node evaluation for anatomical pulmonary segmentectomy.
We do perform standardize mediastinal lymph node dissection for segmentectomy as we would do for lobectomy. Once the systemic mediastinal lymph node dissection is completed then we proceed to segment specific lymph node evaluation.
Right upper lobe segmentectomies
The dissection is started by opening the pleura over the posterior surface and the apex of the lung and over the hilum. Frozen section of level 10 lymph node should be performed by skeletonizing the space over the right main stem bronchus till the inferior margin of the azygous vein. Subsequently, lymph node between apical segment vein and the anterior segmental pulmonary artery branch should be removed. If these lymph nodes are negative, then right upper lobe segmentectomy can be pursued with caution.
Right upper lobe apical segmentectomy (S1)
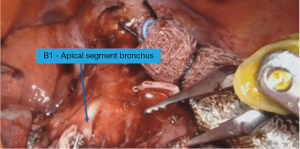
The mediastinal and hilar lymph node dissection is completed as described. The apical vein is transected leading to exposure of the lymph node at the origin of the truncus over the pulmonary artery. If this lymph node is not sent for frozen then it should be done at this point as lobectomy would be a better approach if this lymph node is positive. Subsequently, the truncus branch is dissected till its bifurcation into apical and anterior segment. The lymph node at bifurcation (level 12) is removed. The apical branches are transected leading to the exposure of B1. There are usually smaller lymph node exposed at the meeting site of the three segmental bronchi. The bronchus is cleared to the base and frozen section should be obtained of any suspicious lymph node (Figures 1,2).
Right upper lobe posterior segmentectomy (S2)
Dissection is done in the fissure. The pulmonary artery is exposed. The lymph node over the pulmonary artery is sent for frozen section. Subsequently, the posterior segment vein (V2) arising from the central vein is dissected and transected. Similarly, the pulmonary artery branch (A2) is transected and the fissure is completed. This leads to the exposure of the base of the right upper lobe bronchus, origin of the right upper lobe posterior and anterior bronchi. All the lymph nodes in this area are removed and frozen section performed of a representative lymph node. Then right upper lobe posterior segment bronchus (B2) is transected.
Right upper lobe anterior segmentectomy (S3)
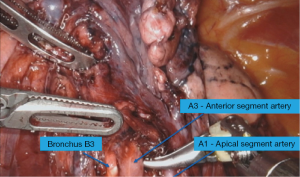
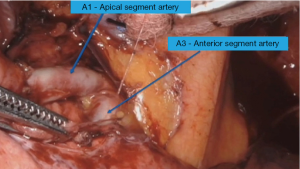
After the completion of the mediastinal and hilar lymph node dissection, the space between the upper lobe and middle lobe vein is dissected. Subsequently, the dissection is done in fissure extending it anteriorly and exposing the central vein of the right upper lobe. The anterior segment branch (V3) is transected leading to the exposure of the bronchus and the artery along with small lymph nodes. It will be appropriate to proceed with segmentectomy if the frozen section is negative otherwise a lobectomy would be a better option (Figure 3)
Left upper lobe segmentectomies
Following left upper lobe segmentectomies are usually performed.
- Left upper lobe upper division segment (S1 + S2 + S3);
- Left upper lobe apicoposterior segment (S1 + S2);
- Lingular segmentectomy (S4 + S5).
A standard left sided mediastinal lymph node dissection is performed. The pleura over the posterior surface of the lung is opened and the level 10 lymph node over the pulmonary artery is sent for frozen section. Subsequently, the lymph node between the upper vein and the main pulmonary artery is evaluated.
Left upper lobe upper division segment (S1 + S2 + S3)
After the completion of the mediastinal and hilar lymph node dissection the fissure is completed posteriorly. The posterior segmental arteries are transected leading to the exposure of the posterior surface of the bronchus and lymph node between lingular and upper division segmental bronchi. The sample should be sent for frozen section. Anteriorly the upper division vein is transected leading to the exposure of the lymph node over the first branch of the pulmonary artery which also should be confirmed to be negative before committing to segmentectomy.
Left upper lobe apicoposterior segment (S1 + S2)
The steps are similar to upper division segmentectomy except that only the apicoposterior vein and bronchus rather than whole upper division and bronchus is transected.
Lingular segmentectomy (S4 + S5)
The mediastinal and hilar lymph node dissection is completed. The dissection is done in the fissure and any suspicious lymph node is sent for frozen. The lingular branches of the artery and vein are transected leading to the exposure of lingular bronchus and lymph nodes in secondary carina which should be sent for frozen before committing to the lingular segmentectomy.
Right and left lower lobe superior and basilar segmentectomy [S6 + S7(8)–10]
Lower lobe segment specific lymph node dissection depends on the targeted segment. Segment 7 is missing on the left. The technique of lower lobe segmentectomy is mentioned in a separate manuscript of this issue. Following is the suggested technique for lymph node dissection.
The pleura over the posterior surface of the lung is opened and the space between the vein and the bronchus is dissected to expose the level 11 lymph node which should be confirmed to be negative.
Any suspicious lymph node over the pulmonary artery and branches should be confirmed to be negative.
Transection of the targeted segmental artery leads to the exposure of the lymph node over the bronchus which should be sent to frozen.
Lower lobe anteromedial (S7 + S8) and posterolateral (S9 + S10) segmentectomy
Similar to common basilar segmentectomy, any suspicious lymph node in the fissure is sent for frozen. Subsequently, the targeted segmental branch is transected which exposes the lymph node over the bronchus which is sent for frozen.
In conclusion, segmentectomy is an acceptable alternate to lobectomy for tumors less than 2 cm.
In addition to the systemic or lobe-specific mediastinal lymph node dissection, a segment specific lymph node dissection should be performed before committing to the segmentectomy to avoid missing nodal upstaging.
Acknowledgments
The authors acknowledge the editorial help of Syeda Sara Abbas.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Video-Assisted Thoracic Surgery for the series “Robotic Segmentectomies”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats-2019-rcs-08). The series “Robotic Segmentectomies” was commissioned by the editorial office without any funding or sponsorship. Alper Toker served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Jun 2019 to May 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. Erratum in: CA Cancer J Clin 2020;70:313. [Crossref] [PubMed]
- De Koning H, Van Der Aalst C, Ten Haaf K, et al. PL02.05 Effects of Volume CT Lung Cancer Screening: Mortality Results of the NELSON Randomised-Controlled Population Based Trial. J Thorac Oncol 2018;13:S185. [Crossref]
- Schuchert MJ, Abbas G, Awais O, et al. Anatomic segmentectomy for the solitary pulmonary nodule and early-stage lung cancer. Ann Thorac Surg 2012;93:1780-5; discussion 1786-7. [Crossref] [PubMed]
- Deng HY, Zhou J, Wang RL, et al. Surgical Choice for Clinical Stage IA Non-Small Cell Lung Cancer: View From Regional Lymph Node Metastasis. Ann Thorac Surg 2020;109:1079-85. [Crossref] [PubMed]
- Kneuertz PJ, Cheufou DH, D'Souza DM, et al. Propensity-score adjusted comparison of pathologic nodal upstaging by robotic, video-assisted thoracoscopic, and open lobectomy for non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:1457-1466.e2. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 353. [Crossref] [PubMed]
- Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6; discussion 1906-7. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Deng HY, Zhou J, Wang RL, et al. Lobe-Specific Lymph Node Dissection for Clinical Early-Stage (cIA) Peripheral Non-small Cell Lung Cancer Patients: What and How? Ann Surg Oncol 2020;27:472-80. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Significance of Lymphadenectomy in Part-Solid Lung Adenocarcinoma: Propensity Score Matched Analysis. Ann Thorac Surg 2018;106:989-97. [Crossref] [PubMed]
- Jensik RJ, Faber LP, Milloy FJ, et al. Segmental resection for lung cancer. A fifteen-year experience. J Thorac Cardiovasc Surg 1973;66:563-72. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87-93. [Crossref] [PubMed]
- Bao F, Ye P, Yang Y, et al. Segmentectomy or lobectomy for early stage lung cancer: a meta-analysis. Eur J Cardiothorac Surg 2014;46:1-7. [Crossref] [PubMed]
- Cao C, Chandrakumar D, Gupta S, et al. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Zhang L, Li M, Yin R, et al. Comparison of the oncologic outcomes of anatomic segmentectomy and lobectomy for early-stage non-small cell lung cancer. Ann Thorac Surg 2015;99:728-37. [Crossref] [PubMed]
- Bedetti B, Bertolaccini L, Rocco R, et al. Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 2017;9:1615-23. [Crossref] [PubMed]
- Lutfi W, Schuchert MJ, Dhupar R, et al. Node-Positive Segmentectomy for Non-Small-Cell Lung Cancer: Risk Factors and Outcomes. Clin Lung Cancer 2019;20:e463-9. [Crossref] [PubMed]
Cite this article as: Abbas G, Raza B, Abbas K, Lamb J, Toker A. Segment-specific lymph node dissection and evaluation during anatomical pulmonary segmentectomy. Video-assist Thorac Surg 2021;6:17.