
Oncologic outcomes in minimally invasive esophagectomy for esophageal carcinoma
Introduction
Esophagectomy continues to play a significant role in the curative treatment for esophageal carcinoma.
A better understanding of surgeon and center surgical volume (1), optimization of patients’ performance status and the reduction of surgical stress with the development of minimally invasive approach, have proven to reduce perioperative morbidity and mortality after esophagectomy. Oncologic esophageal surgery has moved from a transhiatal approach to a transthoracic, 2 or 3 stage operation, favoring better oncologic lymph node dissection over surgical stress secondary to a thoracotomy. In the last 10 years, minimally invasive access has been increasingly adopted, either totally laparoscopic, thoracoscopic assisted, hybrid or robotic surgery.
Minimally invasive surgery [minimally invasive esophagectomy (MIE)] for esophageal cancer has a favorable impact in perioperative morbidity and enhances short term recovery compared to traditional open surgery through thoracotomy [open esophagectomy (OE)]. This report will address whether minimally invasive approaches provide equivalent oncologic outcomes.
Surgical resection remains an integral component of curative intent therapy particularly for esophageal adenocarcinoma (EA). While squamous cell carcinoma (SCC) is the primary cause of esophageal cancer worldwide, EA is the primary cause of esophageal cancer in the Western population with an increasing rate particularly of gastroesophageal junction tumors. New regimens of chemoradiation and chemotherapy have improved disease-free survival (DFS) and overall survival (OS) but curative intent treatment for EA can only be achieved after completing esophagectomy while definitive chemoradiation may be curative for SCC. The key principles of oncologic surgery are essential in esophagectomy for cancer including radical lymph node dissection and negative margins (2-4).
We review here the current standards of MIE esophagectomy for esophageal carcinoma by total laparoscopic, thoracoscopic assisted and robotic approach analyzing perioperative and oncologic results that make this approach comparable to classic OE but with the benefits of reduced surgical stress.
Perioperative morbidity and short-term outcomes of MIE compared to open transthoracic esophagectomy
Historically, esophagectomy has been associated with important rates of morbidity and death. Mortality rate for oesophageal resection in the modern era is close to 5% (5). Pulmonary complications and prolonged hospital stay due to complications are reported in at least half the patients who have OE (6). In 1992, Cuschieri published the first minimally invasive transthoracic esophagectomy (7). Shortly thereafter, others reporting early experiences in MIE, including a case series describing results in nine patients who underwent total laparoscopic esophagectomy (8), suggested the benefits of MIE in terms of shorter postoperative recovery and reduced respiratory complications. Retrospective series directly comparing minimally invasive Ivor Lewis esophagectomy (MIE) and OE have shown improved outcomes in patients undergoing MIE in the early postoperative periods, which were confirmed in a randomized controlled trial (9) and a phase II multicenter trial (10). With the increasing experience with this surgical approach, larger series were reported, particularly in North America. Luketich initially published (11) his experience with 222 patients and later the experience in MIE with a larger group of 1,033 patients (12). This retrospective study, analyzed the perioperative outcomes of MIE as a primary endpoint and compared a minimally invasive McKeown with an Ivor Lewis approach. The 30-day mortality was excellent at 1.68% but increased to 2.8% when all in-hospital mortality was included. Mortality was lower for Ivor Lewis MIE compared to McKeown MIE but the difference was not statistically significant. Among the postoperative morbidity, empyema (6%), anastomotic leak (5%) and gastric tube necrosis (4%) showed no significant differences between both techniques. Recurrent nerve injury was less frequent in the Ivor Lewis MIE group.
The prospective multi-center trial, lead by the same author 3 years later, reported a slightly higher incidence in 30-day mortality and morbidity perioperative mortality of 2.9% (10). Significant complications included anastomotic leak (8.6%), acute respiratory distress syndrome (5.7%), pneumonitis (3.8%), and atrial fibrillation (2.9%).
In comparing by type of MIE approach (total MIE and thoracoscopic assisted) to classic open surgery, Smithers reported less median blood loss, and shorter length of stay (13). Median blood loss for total MIE was 300 compared to 400 mL for hybrid MIE (HMIE) and 600 mL for OE. This report identified lower stricture rates in OE (6.1%) compared to hybrid (21.6%) and total MIE (36%). This finding has not been reported by other authors. Postoperative complications including mortality, were similar among the three groups, with a 30-day mortality rate for open, thoracoscopic assisted and total MIE of 2.6%, 2.3% and 0% respectively.
Biere reported a multicentre, randomised controlled trial wherein patients were randomly assigned via a computer-generated randomisation to open transthoracic (OE) or minimally invasive transthoracic esophagectomy (MIE) (9). Most patients underwent neoadjuvant chemotherapy with paclitaxel and carboplatin. Surgery was performed 6–8 weeks after completion of neoadjuvant therapy. Pulmonary infection within 2 weeks of surgery or occurring during the hospital stay was the primary outcome measure. This was defined by clinical diagnosis confirmed by chest X-ray or CT scan and a positive sputum culture. Secondary outcomes were length of hospital stay and health-related quality of life (HRQOL) measured 6 weeks after surgery. Patients in the MIE group had statistically significant fewer pulmonary infections within 2 weeks of surgery and during the entire hospital stay. As reported by others, hospital stay was significantly shorter for the MIE patients. Also, HRQOL was significantly better in the MIE group, specifically the physical domains of the SF-36 and EORTC QLQ-C30 and the pain and talking components of the OES18.
Similarly, in a randomized clinical trial comparing quality of life after surgery between MIE and OE reported by Maas, MIE was associated with a significantly better HRQOL at 1 year compared to OE for the physical domain of the SF-36 (P=0.003), EORTC QLQ-C30 global health (P=0.004) and OES 18 pain score (P=0.001) (14). A systematic review evaluating HRQOL after MIE compared to open surgery reported that global health, social function and emotional function improved more quickly after MIE, but physical function and symptoms declined similarly after OE and MIE (15). A meta-analysis of nine studies of 1,157 MIE patients and 907 OE patients documented that at 3 months postoperatively patients resected by MIE reported better scores for global quality of life, fatigue and pain compared with OE. However, by 6 months, these differences had resolved (16).
Given the rigors of RCTS, results may not reflect real world experience (17). Database studies may provide more realistic results. The analysis of the Japanese National Clinical Database, compared outcomes between OE and MIE and includes one of the largest patient datasets published on this matter with 24,233 patients analyzed (18). This study evaluated the safety of MIE particularly after neoadjuvant therapy using generalized estimating equations logistic regression analysis. During the study period, the proportion of MIE increased from 33.7% prior to the study to more than 50%. In the early phase of the study MIE was used for early cancers and OE was used for more advanced cancers and those treated with neoadjuvant therapy. However, this evolved over the study period, with increasing use of MIE even for advanced cases. Even including more advanced cancer and neoadjuvant therapy, MIE had similar or fewer complications compared to OE including pulmonary complications, prolonged ventilation >48 hours, unplanned intubation, and sepsis. Reoperation within 30 days was more frequent after MIE (18).
An analysis of the National Cancer Database (NCDB) of esophagectomies performed between 2010 and 2012 used propensity score analysis to compare 2,050 patients treated with OE and 982 treated with MIE. After matching, 977 pairs were evaluated. Short-term outcomes of postoperative complications, length of stay (14 days), 30-day mortality (3%), 90-day mortality (7%) and unplanned readmission (6.5%) were similar between groups (19).
Various meta-analysis have also compared the short term perioperative outcomes of MIE versus OE (20-24), consistently reporting reduced blood loss in MIE. In terms of complication rates, these systematic reviews showed overall reduced complications in MIE (41.5%) compared to OE (48.2%) (Table 1). Anastomotic leak is among the most feared complications after esophagectomy. This complication can reach up to 12% and has not proven to be significantly different when comparing MIE to OE (OR: 1.023, 95% CI: 0.870–1.202, P=0.785).
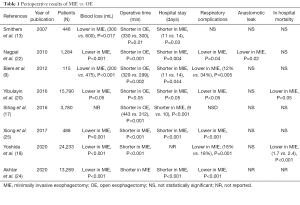
Full table
Oncologic outcomes after MIE compared to OE for esophageal cancer
Because of magnification with the videoscope, minimally invasive techniques provide improved visualisation and may facilitate lymph node dissection. However, a report from the Society of Thoracic Surgeons General Thoracic Database expressed concern regarding the oncologic equivalence of MIE compared to OE (17).
Lymph node dissection, as an oncologic standard for esophageal cancer radical surgery, was previously assessed by studies comparing transhiatal surgery versus transthoracic approach (6,25,26) and is considered an important factor in long-term survival. In an early study published by Hulscher in 2002, there was a trend toward improved long-term survival at 5 years with the extended transthoracic approach compared to transhiatal esophagectomy (6). However, later studies confirmed a survival advantage for transthoracic en-bloc resections OS at 5 years and survival in patients with residual disease after neoadjuvant therapy was 51% overall after en-bloc resection compared to 22% for transhiatal esophagectomy. This difference was statistically significant and for those with residual disease was even more marked (en-bloc 48% compared to 9% for transhiatal).
There is increasing evidence of the importance of radical lymph node resection in improving survival in patients with esophageal cancer (26-30). Regardless of the approach, esophagectomy for cancer must include complete lymphadenectomy of the upper abdominal nodes (left gastric, perigastric, celiac, hepatic and splenic node stations) and the infracarinal thoracic stations (subcarinal, periesophageal and inferior pulmonary ligament nodes). The upper mediastinal node dissection is less frequently completed and is more controversial in the setting of EA, whereas it is standard for SCC. The transthoracic approach, whether MIE or OE allows en-bloc dissection of the intrathoracic nodes and facilitates a R0 resection with respect to the circumferential margin.
The optimum number of lymph nodes required for lymphadenectomy has been controversial. Although guidelines suggest a minimum number, best evidence suggests that the optimum number for survival is dependent on histology, T and N(+) stage. Based on T stage, the optimum number of nodes to be resected for pT1 is 10 for EA and 12 for SCC whether N0 or N positive. For higher T stage cancers, the optimum lymphadenectomy increases with T stage and if nodes are positive. For pT2N0 the optimum number for EA is 15 and 22 for SCC, but 15 for both if 1–6 nodes are positive for either histology. For pT3/T4 N0, 31 nodes are required for EA and 42 for SCC. However, for pT3/T4 N positive this varies with 29 nodes required if 1–2 nodes are involved, 50 nodes if 3–6 nodes are involved and 28 if >6 nodes are involved. The variation in numbers reflects the certainty of nodal staging, i.e., to ensure a patient is really N0, resection of a higher number of nodes is required whereas if more than 6 nodes are positive, accuracy of nodal staging and survival does not improve by resection of more than 28 nodes (28).
The systematic review by Dantoc compared the lymph node dissection by MIE compared to OE (31). This study included seventeen retrospective case-controlled studies, with a total of 1,586 patients analyzed, comparing total minimally invasive approach (MIE), thoracoscopic assisted or HMIE and OE. For MIE, the median (range) number of nodes removed was 16 (5.7–33.90), for HMIE 17 (17–17.15) and for open 10 (3–32.80) and. The difference between the MIE and OE was significant (P=0.03) but not between MIE versus HMIE (P=0.25). In the NCDB study, lymph nodes examined for MIE was 16.3 compared to 14.5 for OE (P<0.001) (19). In terms of resection margins and lymph node yield after neoadjuvant treatment, Biere also demonstrated that MIE is at least comparable to open surgery (9).
Individual institutional series report variable results. Lymph node yield was 30 (IQR: 22–39) in MIE compared to 14 (IQR: 7–19) for OE in the report by Ahmadi (32) whereas Findlay reported inferior lymph node yield in MIE (median 20, IQR: 7–44) compared to open (median 26, IQR: 4–54) or hybrid (median 27.5, IQR: 6–65) (33). The variability in reported lymph node yield likely reflects the diligence of surgeons and perhaps pathologists rather than any superiority of surgical approach.
In the Eastern World, where SCC has a significantly higher incidence than EA, the lymph node dissection of the supracarinal esophagus plays a significant role in achieving an adequate oncologic esophagectomy. In this dissection, there is particular concern in avoiding recurrent laryngeal nerve (RLN) injury, as dissection of both RLN is standard procedure. Better results may be achieved by OE for T3 SCC particularly for the left paratracheal dissection (30). However other studies have demonstrated that the lymph node dissection after MIE is comparable for T1 and T2 SCC (34) while a more recent study reports a good RLN dissection can be achieved by MIE regardless of T stage, without any nerve injury (35). This likely reflects surgeon experience and comfort rather than any difference between surgical approaches.
The ability to achieve an R0 resection overall appears similar (19,21) although an earlier study by Burdall, described a reduction of R1 resections after MIE, at 6.1% compared to 15.6% after OE (36). These results may be confounded by preoperative therapy, specifically radiation.
Comparing perioperative results with MIE to OE after neoadjuvant treatment (either chemoradiation or chemotherapy alone), MIE has proven to be feasible and safe. In a study of 175 patients with SCC treated with neoadjuvant therapy and surgery, MIE was associated with lower blood loss and lower frequency of postoperative complications and no difference in 30- and 90-day mortality (37). Complete pathological response (27.6% vs. 4.8%, P=0.001) and decreased lymph node metastases (25.0% vs. 57.1%, P=0.001) was significantly more frequent after neoadjuvant chemoradiation compared to neoadjuvant chemotherapy alone. This was associated with improved survival although not statistically significant (37).
OS remains the gold standard for oncologic therapy. A study of esophagectomy patients in the Finnish nationwide registry of between 2004 and 2014, matched patients based on sex, age, comorbidity, year of surgery, histology, stage (localized versus locally advanced), neoadjuvant therapy and center volume. In 150 propensity matched pairs, 1-year survival for MIE vs. OE was: 85.3% vs. 74.7% (AHR: 0.53; 95% CI: 0.31–0.99, P=0.0174); at 3 years, 68.7% vs. 55.6% (AHR: 0.62; 95% CI: 0.43–0.91, P=0.0144) and at 5 years 61.8% vs. 51.9% (AHR: 0.68, 95% CI: 0.47–0.97, P=0.0347). In this study, there was no difference in 30- and 90-day mortality (38). In Finland esophagectomy is performed by a small number of surgeons thus variation is between surgeons and centres is reduced. In this context, MIE offers superior survival compared to OE.
In Dantoc’s review, overall 5-year survival was not significantly different between OE (16–57%) compared to MIE 12.5–63% (P=0.93) (31). These results were replicated in a subsequent meta-analysis (5-year survival HR: 0.92; 95% CI: 0.720–1.1.760) (32). In the NCDB review, median survival for MIE was 46.6 months and 48.7 months for OE (19). These results suggest that survival is not dependent on surgical approach but rather components of therapy including neoadjuvant therapy and surgical rigor. In the phase II, multicenter trial investigating MIE, including ECOG, CALGB and ACOSOG centers, estimated 3-year OS was 58.4%, loco-regional recurrence was 6.9% and median survival was not reached (8). See Table 2.
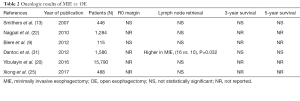
Full table
Robotic surgery for esophageal cancer
Robotic esophagectomy is a relatively new minimally invasive technique. It offers increased magnification, dexterity, and three-dimensional (3D) visual clarity; allowing precise and reproducible performance of en-bloc esophagectomy along with periesophageal tissue in the mediastinum while avoiding injury to the RLN. Kernstine reported the first robotic‐assisted two‐stage, three‐field esophagectomy was in 2004 (39).
Small retrospective studies, including a recent systematic review comparing robotic assisted MIE (RAMIE) to standard thoracoscopic MIE have proven that results of both are comparable (40). There were no significant differences between RAMIE vs. MIE for R0 resection rate, conversion to open, 30‐day mortality rate, 90‐day mortality rate, in‐hospital mortality rate, postoperative complications, number of harvested lymph nodes, operative time, and length of stay. Interestingly, the vocal cord palsy rate was higher in the MIE group compared with RAMIE (OR: 0.5696, P=0.0447). This appears to support stated potential advantages of RAMIE over MIE.
Conclusions
This review of the literature shows the increasing experience in MIE for esophageal carcinoma worldwide, with improved outcomes in terms of enhanced recovery and better postoperative quality of life, without reducing the oncologic rigour of the operation. The oncologic standards achieved by open surgery for EA and SCC, in terms of lymph node dissection and surgical margins, can be achieved after MIE, even after neoadjuvant therapy followed by surgery.
Robotic assisted surgery, with better visual 3D clarity and improved dexterity may offer further improvements, however the economic burden related to this expensive technology remains a limiting factor.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Robert E. Merritt) for the series “Minimally Invasive Esophagectomy for Esophageal Carcinoma” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats-2019-mie-05). The series “Minimally Invasive Esophagectomy for Esophageal Carcinoma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Scheepers JJ, Mulder CJ, Van Der Peet DL, et al. Minimally invasive oesophageal resection for distal oesophageal cancer: a review of the literature. Scand J Gastroenterol Suppl 2006;123-34. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- Swanstrom LL, Hansen P. Laparoscopic total esophagectomy. Arch Surg 1997;132:943-7; discussion 947-9. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Franchetti Y, et al. Minimally invasive esophagectomy: results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study. Ann Surg 2015;261:702-7. [Crossref] [PubMed]
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238:486-94; discussion 494-5. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Smithers BM, Gotley DC, Martin I, et al. Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg 2007;245:232-40. [Crossref] [PubMed]
- Maas KW, Cuesta MA, van Berge Henegouwen MI, et al. Quality of life and late complications after minimally invasive compared to open esophagectomy: results of a randomized trial. World J Surg 2015;39:1986-93. [Crossref] [PubMed]
- Taioli E, Schwartz RM, Lieberman-Cribbin W, et al. Quality of life after open or minimally invasive esophagectomy in patients with esophageal cancer-a systematic review. Semin Thorac Cardiovasc Surg 2017;29:377-90. [Crossref] [PubMed]
- Kauppila JH, Xie S, Johar A, et al. Meta-analysis of health-related quality of life after ninimally invasive versus open oesophagectomy for oesophageal cancer. Br J Surg 2017;104:1131-40. [Crossref] [PubMed]
- Sihag S, Kosinski AS, Gaissert HA, et al. Minimally invasive versus open esophagectomy for esophageal cancer: a comparison of early surgical outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg 2016;101:1281-8; discussion 1288-9. [Crossref] [PubMed]
- Yoshida N, Yamamoto H, Baba H, et al. Can minimally invasive esophagectomy replace open esophagectomy for esophageal cancer? Latest analysis of 24,233 esophagectomies from the Japanese National Clinical Database. Ann Surg 2020;272:118-24. [Crossref] [PubMed]
- Mitzman B, Lutfi W, Wang CH, et al. Minimally invasive esophagectomy provides equivalent survival to open esophagectomy: an analysis of the National Cancer Database. Semin Thorac Cardiovasc Surg 2017;29:244-53. [Crossref] [PubMed]
- Yibulayin W, Abulizi S, Lv H, et al. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta-analysis. World J Surg Oncol 2016;14:304. [Crossref] [PubMed]
- Guo W, Ma X, Yang S, et al. Combined thoracoscopic-laparoscopic esophagectomy versus open esophagectomy: a meta-analysis of outcomes. Surg Endosc 2016;30:3873-81. [Crossref] [PubMed]
- Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 2010;24:1621-9. [Crossref] [PubMed]
- Sgourakis G, Gockel I, Radtke A, et al. Minimally invasive versus open esophagectomy: meta-analysis of outcomes. Dig Dis Sci 2010;55:3031-40. [Crossref] [PubMed]
- Akhtar NM, Chen D, Zhao Y, et al. Postoperative short-term outcomes of minimally invasive versus open esophagectomy for patients with esophageal cancer: an updated systematic review and meta-analysis. Thorac Cancer 2020;1:1465-75. [Crossref] [PubMed]
- Xiong WL, Li R, Lei HK, et al. Comparison of outcomes between minimally invasive oesophagectomy and open oesophagectomy for oesophageal cancer. ANZ J Surg 2017;87:165-70. [Crossref] [PubMed]
- Rizzetto C, DeMeester SR, Hagen JA, et al. En bloc esophagectomy reduces local recurrence and improves survival compared with transhiatal resection after neoadjuvant therapy for esophageal adenocarcinoma. J Thorac Cardiovasc Surg 2008;135:1228-36. [Crossref] [PubMed]
- Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000; discussion 1000-1. [Crossref] [PubMed]
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. [Crossref] [PubMed]
- Kang CH, Kim YT, Jeon SH, et al. Lymphadenectomy extent is closely related to long-term survival in esophageal cancer. Eur J Cardiothorac Surg 2007;31:154-60. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [Crossref] [PubMed]
- Dantoc MM, Cox MR, Eslick GD. Does minimally invasive esophagectomy (MIE) provide for comparable oncologic outcomes to open techniques? A systematic review. J Gastrointest Surg 2012;16:486-94. [Crossref] [PubMed]
- Ahmadi N, Crnic A, Seely AJ, et al. Impact of surgical approach on perioperative and long-term outcomes following esophagectomy for esophageal cancer. Surg Endosc 2018;32:1892-900. [Crossref] [PubMed]
- Findlay L, Yao C, Bennett DH, et al. Non-inferiority of minimally invasive oesophagectomy: an 8-year retrospective case series. Surg Endosc 2017;31:3681-9. [Crossref] [PubMed]
- Ye B, Zhong CX, Yang Y, et al. Lymph node dissection in esophageal carcinoma: Minimally invasive esophagectomy vs open surgery. World J Gastroenterol 2016;22:4750-6. [Crossref] [PubMed]
- Otsuka K, Murakami M, Goto S, et al. Minimally invasive esophagectomy and radical lymph node dissection without recurrent laryngeal nerve paralysis. Surg Endosc 2020;34:2749-57. [Crossref] [PubMed]
- Burdall OC, Boddy AP, Fullick J, et al. A comparative study of survival after minimally invasive and open oesophagectomy. Surg Endosc 2015;29:431-7. [Crossref] [PubMed]
- Tang H, Zheng H, Tan L, et al. Neoadjuvant chemoradiotherapy followed by minimally invasive esophagectomy: is it a superior approach for locally advanced resectable esophageal squamous cell carcinoma? J Thorac Dis 2018;10:963-72. [Crossref] [PubMed]
- Sihvo E, Helminen O, Gunn J, et al. Long-term outcomes following minimally invasive and open esophagectomy in Finland: a population-based study. Eur J Surg Oncol 2019;45:1099-104. [Crossref] [PubMed]
- Kernstine KH, DeArmond DT, Karimi M, et al. The robotic, 2-stage, 3-field esophagolymphadenectomy. J Thorac Cardiovasc Surg 2004;127:1847-9. [Crossref] [PubMed]
- Jin D, Yao L, Yu J, et al. Robotic-assisted minimally invasive esophagectomy versus the conventional minimally invasive one: a meta-analysis and systematic review. Int J Med Robot 2019;15:e1988 [Crossref] [PubMed]
Cite this article as: Devaud NA, Yeung JC, Darling GE. Oncologic outcomes in minimally invasive esophagectomy for esophageal carcinoma. Video-assist Thorac Surg 2021;6:16.


