
Advantages and disadvantages of robotic and uniportal video-assisted thoracoscopic surgery
Introduction
Two hundred years have passed since the advent of pulmonary resection, which was first performed in 1821. Video-assisted thoracoscopic surgery (VATS), which was previously performed as an open surgery prior to being performed endoscopically, has now become the mainstay for surgical pulmonary resection. In recent years, robotic-assisted thoracoscopic surgery (RATS) and uniportal video-assisted thoracoscopic surgery (U-VATS) have been reported as new surgical approaches. RATS, which is an endoscopic procedure that uses robotic systems, was first described by Melfi et al. in 2002 and is now widely used worldwide (1). The robotic system used most commonly at present is the da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA, USA). The advantages associated with robotic-assisted surgery using the da Vinci surgical system include a three-dimensional surgical view, the elimination of physiological tremors, and the ability to perform surgical manipulation in a natural orientation because of the presence of forceps that move in the same manner as human wrist joints. Its disadvantage is the absence of tactile sensation. As a new surgical approach, U-VATS has been used more widely, together with robotic surgery, mainly in Asia and Europe. Unlike multiportal VATS, which is performed by inserting ports into three to four incisions, U-VATS is a surgical procedure that is performed using only one incision of 4 cm or less (2). U-VATS was reported as a partial pulmonary resection procedure by Rocco et al. in 2004 (3). After the first U-VATS lobectomy was reported by Gonzalez et al. in 2011, the procedure has been used worldwide (4). The advantages associated with U-VATS include the expectations of a lower level of postoperative pain because of the single incision and a faster recovery. Its disadvantage is that all surgical instruments are inserted via a single incision, which may limit surgical manipulation and reduce surgical safety and accuracy.
In this article, reports of RATS and U-VATS are reviewed and the advantages and disadvantages associated with the two surgical techniques and their respective uses are discussed. Only the study by Yang et al. (5) has directly compared RATS with U-VATS. Thus, we reviewed RATS and UVATS based on studies in which RATS and VATS and U-VATS and VATS were compared.
Current status of RATS
Because RATS is a novel surgical technique, various port placements have been reported; however, in general, three to five ports are prepared for the procedure (6-8). One of the disadvantages of RATS is that the number of ports is high, even compared with multiportal VATS, which is performed manually by human hands. Reports of comparisons of RATS to open thoracotomy indicate that the former can be performed safely; is associated with lower morbidity and mortality; and affords a reduction in postoperative hospital stay, a greater number of lymphadenectomies, and improved postoperative quality of life (7); however, RATS has been reported to have longer operative times (9).
To date, no prospective studies have compared RATS and VATS, and mixed results have been reported for the comparisons of these two techniques. The advantages associated with RATS vs. VATS reportedly include a higher number of lymphadenectomies, its similarity to open thoracotomy, less complications, less blood loss, a shorter hospital stay, use of fewer analgesics, and an earlier return to daily activities (10-19). Conversely, the disadvantages associated with RATS compared with VATS reportedly include longer operative times, higher costs, greater postoperative pain, and a higher incidence of postoperative pulmonary leaks (14-19). In terms of operative times, the specific action of docking the patient cart in RATS may prolong its operative time (15), which may be one of its drawbacks in terms of effective utilization of operating rooms, as it will increase their occupancy time. Regarding the incidence of postoperative complications, we reported previously that RATS had a significantly lower rate of complications compared with VATS (20); however, several studies have reported that RATS is associated with more intraoperative blood loss and more postoperative pulmonary leaks (19). Regarding vascular injuries, Cerfolio et al. reported these injuries in 15 (2.4%) out of 632 patients undergoing robotic-assisted procedures in 2016, and concluded that vascular injuries can be safely managed even with robotic procedures (21). In terms of pain, although the presence of a forceps joint was expected to reduce compression on the intercostal nerves and decrease pain in RATS, an increased level of pain has been reported (18). The costs of performing RATS were reportedly higher than the costs of performing VATS (16,17). This cost increase is likely attributable to the robot-specific consumables used in RATS (10). Regarding long-term results, in 2012, Park et al. reported a multicenter study involving 325 patients in which the 5-year survival rate was 80% (91% for Stage IA, 83% for Stage IB, and 49% for Stage II), showing a favorable outcome. Data reportedly showed that RATS was safe and efficient and was associated with a similar survival rate (22). In 2017, Yang et al. compared the long-term results of open thoracotomy, VATS, and robotic surgery and found that minimally invasive approaches to lobectomy for clinical stage I non-small lung cancer resulted in similar long-term survival compared with thoracotomy. The use of VATS and robotics was also reportedly associated with a shorter length of hospital stay, and the robotic approach resulted in greater lymph node assessment (11). In 2018, Cerfolio et al. reported a large study of long-term survival after RATS, with 5-year survival results according to stage being reportedly comparable to those of conventional techniques, with 83% 5-year survival rates for Stage IA disease, 77% for Stage IB, 68% for Stage IIA, 70% for Stage IIB, 62% for Stage IIIA, and 31% for Stage IIIB (23) (Table 1).
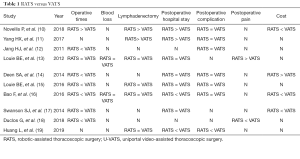
Full table
Issues pertaining to the evaluation of robotic-assisted surgery at this point in time include the high number of ports, the inefficiency in utilizing medical resources because of the prolonged operative time and consequent prolongation of operating room occupancy time, the high cost, and the insufficient demonstration of utility for the patient commensurate with the higher costs.
A randomized trial is currently underway to compare the early results of robotic-assisted surgery with those of VATS for Stage I and II lung cancer (24). Future evaluations of the utility of robotic surgeries via additional multicenter randomized controlled studies are warranted. Robotic systems are being improved constantly, and uniportal robotic systems are also currently being developed. There is a need to investigate further the utility of robotic systems using the latest models and instruments, including assessments of safety and pain associated with robotic surgery, complication rates, accurate diagnosis of lymph nodes, and long-term results.
Current status of U-VATS
U-VATS is a surgical method that uses a single incision of 4 cm or less. The history of uniportal surgery begins with a report of the use of a single incision for thoracic sympathectomy performed for palmar hyperhidrosis between 1990 and 1992 (25). Since the report by Gonzalez-Rivas et al. of a U-VATS lobectomy from a single incision in 2011, this technique has spread mainly in Asia and Europe and, in recent years, has also been applied during more sophisticated surgical techniques, such as sleeve resection, segmental resection, and carinaplasty (26-28). Currently, this method appears to be the most minimally invasive surgical approach for lung cancer. If the method does not differ from conventional thoracoscopic surgery, uniportal surgery may be a quite useful procedure for patients, because of its minimal invasiveness and cosmetic nature.
Comparisons between U-VATS and VATS have revealed that uniportal surgery results in less intraoperative blood loss (29). U-VATS has also been reported to cause less postoperative pain, although there are some reports of an absence of differences in postoperative pain between these techniques (30). U-VATS has also been reported to be superior to VATS in terms of the incidence of post-thoracotomy pain syndrome (31). As only one incision is used in U-VATS, this procedure imparts less damage than does VATS, which requires multiple incisions. Thus, reduced intraoperative blood loss and postoperative pain are to be expected. A disadvantage reportedly associated with U-VATS compared with VATS is the longer duration of lymphadenectomy (32). However, according to a study, with U-VATS, results for the extent of lymph node dissection and the number of dissected lymph nodes were equal to or better than those with VATS (29,33-39). In addition, Ismail et al. reported that U-VATS allows for safe and effective radical lymphadenectomy comparable to other minimally invasive techniques (33). Because U-VATS is a novel surgical technique, few studies have reported its long-term outcomes. Uniportal surgery can be accomplished within a similar duration as multiportal VATS if the surgeon is familiar with the procedure; for patients, it is thus reasonable to expect that, as the damage to the intercostal space is limited to one location, postoperative pain, the extent of intercostal nerve injury, and the incidence of post-thoracotomy pain would be reduced in the case of uniportal surgery. The proof of the utility of uniportal surgery is not sufficient at present; thus, its utility compared with VATS is required to be demonstrated in a multicenter randomized control study in the future (Table 2).
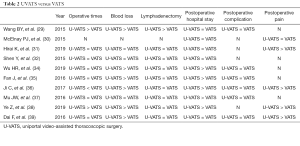
Full table
Discussion
Few reports have compared directly uniportal resection with robotic-assisted pulmonary resection. In the study reported by Yang et al., the superiority of RATS vs. U-VATS was observed based on less intraoperative blood loss and more lymphadenectomy sites; conversely, the superiority of U-VATS vs. RATS was observed based on the shorter period of chest drain placement. Yang et al. reported an absence of differences in operative time, period of hospital stay, postoperative analgesic use, and incidence of complications (5). RATS may be superior to U-VATS in cases that require more challenging surgical manipulation, because of its superior maneuverability. In lung cancer surgery, the presence of a joint that moves as well as the human wrist joint in lymphadenectomy, which requires manipulation in deep sites, allows the performance of the procedure in the same manner as open surgery. This is an advantage over uniportal surgery, which is associated with limited maneuverability. Moreover, limitations in suture manipulation have arisen during uniportal surgery. Uniportal bronchoplasty and angioplasty have been reported; however, uniportal procedures that limit the orientation of the instruments result in very difficult procedures. According to Veronesi et al., robotic-assisted surgery for advanced lung cancer facilitates lymph node resection, and precise lymph node resection can lead to accurate diagnoses of patients requiring adjuvant chemotherapy, thus allowing rapid prognostic improvement in these patients (40). We also performed robotic-assisted graft replacement of innominate veins, which is not considered feasible via manual VATS using human hands, in surgeries for thymic carcinoma (41). Robotic-assisted surgery is a minimally invasive surgical technique in which highly challenging procedures can be achieved that are not feasible with manual VATS using human hands, which may be of clear benefit for some patients.
Conversely, there is no doubt that U-VATS is associated with a lower burden on patients as the surgery is performed using only one incision. RATS is also a minimally invasive procedure compared with open thoracotomy; however, currently, more port insertions are required (similar to, or more than, those required for VATS) and the burden on patients may be comparable to that of VATS (31). If uniportal surgery is safe and can be performed with adequate accuracy, including for lymph node resection, this surgical technique can be useful for patients. Thus, at present, uniportal surgery may be a beneficial surgical procedure for patients as a minimally invasive procedure in those for whom the required procedure is not associated with high difficulty, provided that the surgeon has adequate skill, while robotic surgery may be useful for more challenging surgical procedures. In the future, the safety, degree of invasiveness, and oncological long-term results of these new surgical methods should be investigated and compared with those of conventional surgical methods, to assess their benefits.
In recent years, a variety of new systems have been developed for robotic surgical systems. Uniportal robotic surgical systems and robotic systems with tactile sensation are also being developed (42,43). Procedures performed manually with human hands have limitations; however, robotics will continuously be developed in the future. Robotic surgery, aimed at high accuracy, and uniportal surgery, aimed at minimally invasive surgery, will likely eventually be fused.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kazuo Yoshida) for the series “Robotic VS Uniportal VATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats-20-34). The series “Robotic VS Uniportal VATS” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Melfi FM, Menconi GF, Mariani AM, Angeletti CA. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-68. [Crossref] [PubMed]
- Bertolaccini L, Batirel H, Brunelli A, et al. Uniportal video-assisted thoracic surgery lobectomy: a consensus report from the Uniportal VATS Interest Group (UVIG) of the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;56:224-29. [Crossref] [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS Wedge Pulmonary Resections. Ann Thorac Surg 2004;77:726-28. [Crossref] [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Yang S, Guo W, Chen X, et al. Early outcomes of robotic versus uniportal video-assisted thoracic surgery for lung cancer: a propensity score-matched study. Eur J Cardiothorac Surg 2018;53:348-52. [Crossref] [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-46. [Crossref] [PubMed]
- Veronesi G. Robotic lobectomy and segmentectomy for lung cancer: results and operating technique. J Thorac Dis 2015;7:S122-30. [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Novellis P, Bottoni E, Vonlaz E, et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: comparison of costs and outcomes at a single institute. J Thorac Dis 2018;10:790-98. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-Term Survival Based on the Surgical Approach to Lobectomy for Clinical Stage I Non-Small Cell Lung Cancer: Comparison of Robotic, Video Assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Jang HJ, Lee HS, Park SY, et al. Comparison of the early robot-assisted lobectomy experience to video-assisted thoracic surgery lobectomy for lung cancer: a single-institution case series matching study. Innovations (Phila) 2011;6:305-10. [Crossref] [PubMed]
- Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and mobidity with compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598-604. [Crossref] [PubMed]
- Deen SA, Wilson JL, Wilshire CL, et al. Defining the Cost of Care for Lobectomy and Segmentectomy: A Comparison of Open, Video-Assisted Thoracoscopic, and Robotic Approaches. Ann Thorac Surg 2014;97:1000-7. [Crossref] [PubMed]
- Louie BE, Wilson JL, Kim S, et al. Comparison of VATS and Robotic Approaches For Clinical Stage I and II NSCLC Using the STS Database. Ann Thorac Surg 2016;102:917-24. [Crossref] [PubMed]
- Bao F, Zhang C, Yang Y, et al. Comparison of robotic and video-assisted thoracic surgery for lung cancer: a propensity-matched analysis. J Thorac Dis 2016;8:1798-803. [Crossref] [PubMed]
- Swanson SJ, Miller DL, McKenna RJ, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: Results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [Crossref] [PubMed]
- Duclos G, Charvet A, Resseguier N, et al. Postoperative morphine consumption and anaesthetic management of patients undergoing video-assisted or robotic-assisted lung resection: a prospective, propensity score-matched study. J Thorac Dis 2018;10:3558-67. [Crossref] [PubMed]
- Huang L, Shen Y, Onaitis M. Comparative study of anatomic lung resection by robotic vs. video-assisted thoracoscopic surgery. J Thorac Dis 2019;11:1243-50. [Crossref] [PubMed]
- Nakamura H, Suda T, Ikeda N, et al. Initial results of robot-assisted thoracoscopic surgery in Japan. Gen Thorac Cardiovasc Surg 2014;62:720-5. [Crossref] [PubMed]
- Cerfolio RJ, Bess KM, Wei B, et al. Incidence, Results, and Our Current Intraoperative Technique to Control Major Vascular Injuries During Minimally Invasive Robotic Thoracic Surgery. Ann Thorac Surg 2016;102:394-9. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Cerfolio RJ, Ghanim AF, Dylewski M, et al. Yhe long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Veronesi G, Cerfolio R, Cingolani R, et al. Report on First International Workshop on Robotic Surgery in Thoracic Oncology. Front Oncol 2016;6:214. [Crossref] [PubMed]
- Nesher N, Galili R, Sharony R, et al. Videothorascopic sympathectomy (VATS) for palmar hyperhidriosis summary of a clinical trial and surgical results. Harefuah 2000;138:913-6. [PubMed]
- Federici S, Gonzalez M. Uniportal video-assisted thoracoscopic right upper sleeve lobectomy. Multimed Man Cardiothorac Surg 2019. Available online: https://mmcts.org/tutorial/1371
- Hernandez-Arenas LA, Purmessur RD, Gonzalez RD. Uniportal video-assisted thoracoscopic segmentectomy. J Thorac Dis 2018;10:S1205-14. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections. Eur J Cardiothorac Surg 2016;49:i6-16. [PubMed]
- Wang BY, Liu CY, Hsu PK, et al. Single-incision Versus Multiple-incision Thoracoscopic Lobectomy and Segmentectomy. A Propensity-matched Analysis. Ann Surg 2015;261:793-99. [Crossref] [PubMed]
- McElnay PJ, Molyneux M, Krishnadas R, et al. Pain and recovery are comparable after either uniportal or multiportal video-assisted thoracoscopic lobectomy: an observation study. Eur J Cardiothorac Surg 2015;47:912-5. [Crossref] [PubMed]
- Hirai K, Usuda J. Uniportal video-assisted thoracic surgery reduced the occurrence of post-thoracotomy pain syndrome after lobectomy for lung cancer. J Thorac Dis 2019;11:3896-902. [Crossref] [PubMed]
- Shen Y, Wang H, Feng M, et al. Single- versus multiple-port thoracoscopic lobectomy for lung cancer: a propensity-matched study. Eur J Cardiothorac Surg 2016;49:i48-53. [PubMed]
- Ismail M, Nachira D, Swierzy M, et al. Lymph node upstaging for non-small cell lung cancer after uniportal video-assisted thoracoscopy. J Thorac Dis 2018;10:S3648-54. [Crossref] [PubMed]
- Wu HR, Liu CQ, Xu MQ, et al. Systematic mediastinal lymph node dissection outcomes and conversion rates of uniportal video-assisted thoracoscopic lobectomy for lung cancer. ANZ J Surg 2019;89:1056-60. [Crossref] [PubMed]
- Fan J, Yao J, Wang Q, et al. Safety and feasibility of uniportal video-assisted thoracoscopic surgery for locally advanced non-small cell lung cancer. J Thorac Dis 2016;8:3543-50. [Crossref] [PubMed]
- Ji C, Xiang Y, Pagliarulo V, et al. A multi-center retrospective study of single-port versus multi-port video-assisted thoracoscopic lobectomy and anatomic segmentectomy. J Thorac Dis 2017;9:3711-18. [Crossref] [PubMed]
- Mu JW, Gao SG, Xue Q, et al. A propensity matched comparison of effects between video assisted thoracoscopic single-port, two-port, and three-port pulmonary resection on lung cancer. J Thorac Dis 2016;8:1469-76. [Crossref] [PubMed]
- Ye Z, Zhang B, Chen Y, et al. Comparison of Single Utility Port Video-Assisted Thoracoscopic Surgery (VATS) and Three-Port VATS for Non-Small Cell Lung Cancer. Oncol Lett 2019;18:1311-17. [Crossref] [PubMed]
- Dai F, Meng S, Mei L, et al. Single-port Video-Assisted Thoracic Surgery in the Treatment of Non-Small Cell Lung Cancer: A Propensity-Matched Comparative Analysis. J Thorac Dis 2016;8:2872-78. [Crossref] [PubMed]
- Veronesi G, Novellis P, Difrancesco O, et al. Robotic assisted lobectomy for locally advanced lung cancer. J Vis Surg 2017;3:78. [Crossref] [PubMed]
- Suda T, Nagano H, Kawai H, et al. Subxiphoid Robotic-Assisted Thymectomy With Vascular Prosthetic Replacement. Semin Thorac Cardiovasc Surg 2019;S1043-0679(19)30164-9.
- Gonzalez-Rivas D, Ismail M. Subxiphoid or subcostal uniportal robotic-assisted surgery: early experimental experience. J Thorac Dis 2019;11:231-39. [Crossref] [PubMed]
- Stephan D, Salzer H, Willeke F. First Experiences with the New Senhance® Telerobotic System in Visceral Surgery. Visc Med 2018;34:31-6. [Crossref] [PubMed]
Cite this article as: Nagano H, Suda T. Advantages and disadvantages of robotic and uniportal video-assisted thoracoscopic surgery. Video-assist Thorac Surg 2021;6:14.


