What have we learned in the process of setting up and running the PulMiCC (Pulmonary Metastasectomy in Colorectal Cancer) randomised controlled trial?
Introduction
In 2004 NICE, the body that advises the National Health Service in England and Wales, recommended that lung metastasectomy should be offered to selected patients. At the time the acronym stood for National Institute for Clinical Effectiveness. The guidance put liver and lung together in the same recommendation, although for neither was there randomised controlled trial (RCT) evidence, which is the usual standard. Instead a single observational study was cited, which was about liver metastasectomy, and did not provide any evidence about lung metastasectomy (1).
Signaling the need for better evidence
Alerted by this, a first step was to publicly question the NICE guidance. An article asking if there was adequate evidence on which to base the practice of lung metastasectomy was published in the British Medical Journal in 2007 (2). It might have revealed unpublished trials, or other forms of evidence, or generated support. Instead there was an immediate challenge from a group of liver surgeons, including the surgeon who had chaired the NICE Guideline Development Group (3). The BMJ announcement had identified opposition, but did not unearth better evidence concerning lung metastasectomy. There was simply a restatement that metastasectomy “can improve five-year survival from close to zero to over 30%”.
Defining the scope
Let us be clear from the outset, that we are concerned in this review with lung metastasectomy. The only paper cited by NICE in support was a retrospective analysis of selected cases of liver metastasectomy, without a comparator (4). The predilection of colon cancer to metastasise to the liver first, is due to the midgut venous drainage to the liver. Hence there is, at least, a clinical rationale for rescuing patients who have had a locally curative bowel resection, by removing their liver metastases; but this rationale does not apply to the lung. The liver regenerates after surgery—the lungs do not. The history of the practice of liver resection is well documented (5). Suggestions for clinical trials were made (6), and contested, with surgeons making their own assumptions about what an ethics committee might say. No trial was ever done even though it was pointed out that, if true, a difference between zero and 30% could be determined with as few as 36 patients (6). Liver metastasectomy is vigorously promoted by those involved in the practice (7) but that is not the same as having evidence for benefit. There may be general guiding principles in the management of cancer, but we wanted to find the evidence, specifically for the clinical utility of lung metastasectomy, in the case of patients with advanced colorectal cancer (CRC).
The pre-PulMiCC systematic review
A research proposal of any kind should start by asking the question “What do we know already?”. The search for an answer should go beyond pulling out collected papers from the filing cabinet, as was the tradition for many years in the writing of clinical reviews. In such reviews, the cited papers had usually been collected with a likely feature in common—that they supported, and reaffirmed, the proposer’s prior beliefs. The search for papers should therefore be systematic and unbiased. It should seek to discover not just the conclusions of ‘experts’ in the field, but the evidence from which fresh and unbiased conclusions can be derived.
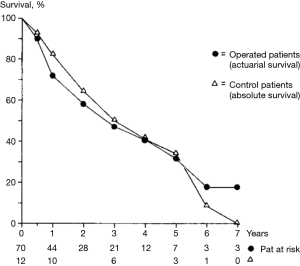
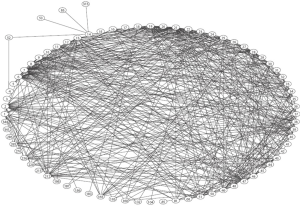
A literature search up to 2010, looking for all that had been written on lung metastasectomy for colorectal cancer (CRC) found neither RCTs nor usable comparator data (8). There was one paper from 1980 using historical controls. The survival of patients who would have been eligible but did not have metastasectomy, in the era preceding its adoption, was used for comparison with a series of 70 patients operated from 1961 to 1978. Five-years survival was similar in the two groups at over 30% (9) (Figure 1). A citation network analysis showed that, while those reporting uncontrolled clinical series of lung metastasectomy cited others believing in the practice, this paper questioning effect of metastasectomy was almost completely ignored (10) (Figure 2).
Analysis of the evidences by quantitative synthesis
Work continued on the systematic review. The search found 101 papers on the subject of lung metastasectomy for CRC. We excluded papers with a mixture of primary cancer sites, other metastatic sites, or non-surgical ablative techniques. This narrowed the papers down to 51, with extractable data on 3,504 patients. There were about 20 reports in the 1970s and 1980s and about 30 in the 1990s. They were markedly formulaic, presenting the sort of data easily garnered from the patients’ files and institutional records. Data on lung function was given in only five which was notable, considering they were operating on the patients’ lungs. None gave any account of symptoms. The characteristics of the patients and their metastases were fairly consistent and easily summarised in round numbers: roughly 60% had a solitary metastasis, 50–60% were male, 60% had non-elevated carcinoembryonic antigen (CEA), 40–60% had rectal cancer, 30% had previous liver metastasectomy, the interval since primary cancer resection was 20 to 40 months, and 50–70% had died within five years. None of these publications formally addressed the question of what survival would have been without metastasectomy. They didn’t even hazard a guess.
The textual narrative and the data selected for presentation in these reports were all about survival (8). These patients had asymptomatic metastases, in all instances, as far as can be determined from the reports. Symptoms attributable to the metastases would almost certainly identify the metastasis as not removable with curative intent.
International collaboration
The European Society of Thoracic Surgeons’ Lung Metastasectomy Project published a supplement to the Journal of Thoracic Oncology in the same year (12). It was clear that the beliefs and practices of the participants were in line with the findings of the systematic review. So that the PulMiCC trial would match ‘Real World’ clinical practice, the criteria for inclusion and exclusion were liberal and allowed for surgeons to follow their usual approach to this disease. We also knew from the many observational studies and systematic reviews that the preoperative characteristics influenced survival. These were the number of metastases, the interval since primary CRC resection, the TNM stage of the primary cancer, the CEA level, and prior liver metastasectomy. Randomisation included minimisation for these factors, plus age and sex, to ensure balance between the trial arms. The ESTS Project included an announcement of the PulMiCC RCT which launched in March of that year (13). It was going to take some time, so we can digress on to other matters and imagine PulMiCC running in the background.
A “moving target”?
One of the papers in the supplement was given the title “Pulmonary Metastasectomy: A Moving Target”. Looking at that again with the passage of time, and in light of the PulMiCC trial, the practice of CRC lung metastasectomy has been remarkably static. The criteria set out by Thomford 55 years ago are pretty much as they are now (14). The favourable and unfavourable factors were shown in the International Registry of Lung Metastases 1997 (15). They were confirmed by a number of authors including Pfannschmidt et al. (16-18). The meta-analysis by Gonzalez et al. was valuable in deriving hazard ratios for the adverse preoperative prognostic factors—multiple metastases, short interval since primary resection and elevated carcinoembryonic antigen (19). Five-year survival has been around 40% throughout. The clinical practice of metastasectomy, as reported in the literature, has not changed materially, although there has been widespread, but not universal adoption of video-assisted thoracic surgery. It has been suggested that new markers or genomic analysis will help target practice. That appears to be at an exploratory stage (20) and had no impact on practice as seen during PulMiCC recruitment.
Randomised trials: “where there’s a will there’s a way”
“But left to their own devices doctors were inevitably likely (even if unconsciously so) to select certain types of patients up front, then judge the effects of a [treatment] on this highly skewed population using subjective criteria, piling bias on bias” (21). So wrote Siddartha Mukherjee in The Emperor of all Maladies. Among patients who have had a resection for CRC, only about 1 in 50 has a lung metastasectomy (22), and the opportunity select the natural winners, and to attribute their survival to the operation, may explain the trust that has built up in the clinical effectiveness of metastasectomy.
During the Covid-18 pandemic the need for randomised trials of treatment clearly had wide acceptance. People worked collaboratively. Funds were made available. Ethics committees did their work at speed, meeting on line, and expediting decisions. The need for trials was self-evident. Amidst all the supportive treatments given, the desperate attempts to keep the worst affected alive, and scratch teams working to as yet non-existent protocols, how could anyone discern the benefit of any individual component of care? For example, the prior positions with respect to dexamethasone were contradictory—would it do more harm than good? The RECOVERY trial allowed the signal of benefit from dexamethasone, to be heard above the noise of the traffic (23). Implicit in all of this was the need for controlled trials to test treatments. But the PulMiCC trial, launched in 2010, was a much slower process; there was nothing more the trial team could do but to continue to encourage recruitment and publish interim progress reports (24).
Randomised trials that altered practice
Vinay Prasad, like Mukherjee, is a radical thinker in cancer research. Between 2001 and 2010, RCTs published in the New England Journal of Medicine, had contradicted 146 medical practices (25). In 2015 he co-authored a systematic review and meta-analysis of treatments for advanced CRC. It was noted that while there had been many trials of systemic treatments there had been none for metastasectomy, and yet the practice was increasing (26). The authors dared to imply that the apparent association of better survival and metastasectomy might be no more than that. It might even be reverse causation: it is not that the metastasectomy makes them live longer, but that longer living people provide more opportunities for surgeons to perform operations on them. That is a rather chilling thought.
Earlier detection of metastases has not improved survival
Another piece of research added weight to the need for PulMiCC—the recovery of the CEA Second Look (CEASL) trial (27). A means of achieving higher rates of metastasectomy is to detect metastases earlier, in the hope of a better chance to remove all residual disease. When the biomarker CEA became available in the 1970s this promised a means of earlier detection. The CEASL trial was run from 1982 to 1993 recruiting 1,447 patients. CEA detected recurrence about a year earlier than presentation with symptoms, or clinical follow-up. Unfortunately, there was no survival benefit demonstrated, but in fact a small (statistically non-significant) detrimental effect (28). Even more unfortunate was that this disappointing result led to the trial data being shelved, unpublished for 20 years (29). A subgroup of the PulMiCC investigators retrieved the data, updated the survival from national death certification, and published the long term results with the same conclusion (27). Since CEASL there have been 16 further randomised trials, testing progressively more intensive monitoring, with newer methods such as CT scanning, and increasing frequency of investigations. The more intensive monitoring again had the power to detect active CRC earlier, but no survival advantage ensued (30,31). The Mayo Clinic oncologist Charles Moertel had written in 1978 “In view of our limited therapeutic accomplishments under these circumstances, the only demonstrable product of this great expenditure for most patients would seem to be the needless anxiety produced by premature knowledge of a fatal disease.” (32). Those remarks may be regarded as unacceptably fatalistic in these days but there are plenty of well-informed men who do not want a PSA test (prostate specific antigen) based on the available evidence.
The PulMiCC trial process
Recruitment into PulMiCC was slow and became slower to reach a near standstill in 2015. PulMiCC Stage 1 invited people with lung metastases from CRC to sign informed consent for assessment of their suitability for metastasectomy. Those who met the trial’s criteria for randomisation were then invited to sign further informed consent for Stage 2, the RCT. The two-stage method worked well in the MARS trial of radical surgery for mesothelioma (33) but in PulMiCC, progression to randomisation in Stage 2 was never adequate. At a conversion rate below 20%, PulMiCC was recruiting too few patients to be convincing. Professor Baum, the chair of the independent Data Monitoring and Ethics Committee (DMEC), asked for an investigation.
Reasons for failure to randomise
Randomisation into surgical and other interventional trials is notoriously difficult. It is made particular difficult if it comes across as ‘something versus nothing’ rather than a ‘drug A versus drug B’ comparison. Because of that difficulty, we tried to ensure that the eligibility for randomization was decided in the multidisciplinary team (MDT or Tumour Board). Sometimes surgeons say that they, and only they, can explain an operation to the patient. This misses the point. The first conversation with a patient, who may be a candidate for the trial, is to introduce uncertainty, which is the reason why the trial is being done, not to explain the details of an operation which 50% of them will not have. The two options must be presented even handedly along with the uncertainty as to which offers the best chance of survival. Surgeons find admitting uncertainty uncomfortable. In “Real World” practice, the MDT discusses each patient, and takes a view on whether the management of the patients should be chemotherapy, radiation therapy, surgery, some combination of treatments, or none. Surgeons only see patients after the agreement of the MDT (which the surgeon usually attends) that an operation is the preferable treatment and the patient agrees. That is the way it should happen in a randomised trial. Two conversations are required. The first is with a neutral person, usually a trial-trained nurse, who should collect consent to participate in the trial and be randomised. Then the surgeon meets patients randomly assigned to have an operation and can focus on the job of building trust and confidence (34).
Finding that far too few of the patients who had consented to be in Stage 1 (evaluation) were progressing to Stage 2 to be randomised, the Trial Management Group requested the Principal Investigators, of the three British sites with the largest enrolment, to find out the reasons for not randomising. They provided answers on all their 155 participants. There were 41 people who made their own decision to have, or not have, lung metastasectomy. They chose in more or less similar numbers (22:19). This reveals a fair degree of group equipoise. But there were 78 patients who could have been randomised, but the MDT took the decision for them: 77/78 (99%) had metastasectomy (35). It only requires one to say highly emotive and value laden words such as “we shouldn’t deny the patient the chance of cure” to make randomisation impossible. That usurps the patients preference to be in a trial. In the event, the trial results indicate that these patients, as a group, would have done just as well if they had been spared surgery. It was estimated that at least 56% of eligible patients were lost because their willingness to be randomised was overridden, thus undermining the trial recruitment.
PulMICC publications
PulMiCC was published in 2019 with survival data on 65 patients and their quality of life (35). The full report in 2020 gave survival data in all 93 randomised patients with 18 months additional follow-up (36) (Figure 3). There were no perioperative deaths. No control patients crossed over to intervention, but three patients in the metastasectomy arm did not have the operation. Chemotherapy and ablative treatments were used similarly in both arms. There was no imbalance in subsequent treatments.
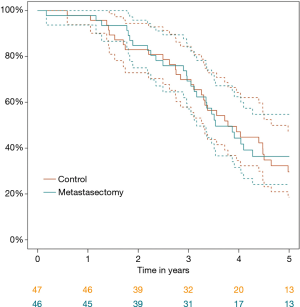
The key message is that there was no survival benefit and this is not because the trial was too small to detect it, but because the control survival was much better than widely believed, a finding which we anticipated in our power calculation for non-inferiority. Apart from the expected dip in patient reported outcome measures (PROMs) in the three months following surgery in the metastasectomy arm, there was no difference in QoL. The common fallback justification of offering surgery for psychological benefit, was not supported by evidence. Assessment of patients’ well-being using the EuroQoL groups EQ-5D-3L questionnaire showed no difference in Health Utility between the groups.
New information may be evident first in control groups
There are now three RCTs of local interventions to treat metastases CLOCC, SABR-COMET and PulMiCC (36-38). All were hard to complete and recruited modest numbers: 119, 99 and 93 respectively. The RCTs tested different treatments: RFA, SABR and surgical metastasectomy. Targets were liver, any site, and lung but the control groups were patients with metastases, not being locally treated so despite heterogeneity in the active arms there was consistency among the controls. Treated patients had five-year survivals of 43%, 46% and 38%. These are in accordance with follow-up studies and are ‘real world’ results.
Pause there. Let’s go back to how PulMiCC started. Asking for the evidence, we were directed to a 2004 NICE document: Improving outcomes in colorectal cancer: manual update. This stated “Surgery for patients with metastases confined to the liver or lung can be curative… it can improve five-year survival from close to zero to over 30%.” Now we have evidence showing that the control group survival is not “close to zero”. RCT patients who did not receive local treatments for their metastases had five years survivals of 30%, 24% and 29% (36-38). The Society of Thoracic Surgeons consensus statement in 2019 (39) found that “metastatic disease survival is assumed to be zero, a contention not supported by the literature.” The control groups of CLOCC and PulMiCC, together provide 27 five-year survivors among 106 patients. That is 29% (95% confidence intervals: 22–37%) robustly contradicting the zero-survival assumption.
A return to realism
Without control data it may be supposed that a metastasectomy operation, performed five years ago, deserves the credit for the life of the patient in front of you; in most instances that is probably not the case. CLOCC authors somewhat disingenuously wrote in 2012 “The study shows that local tumor ablation by RFA in combination with systemic therapy results in an excellent survival, which however was also achieved in the control arm.” (37). SABR-COMET authors were also surprised: “The better-than-expected survival in both groups suggests that oligometastatic cancers behave more indolently than previously appreciated” (38). Åberg told them that in 1980, published prominently in the Annals of Thoracic Surgery, but he was ignored. It is quite likely that there are occasional patients in the tail of the survival curves, in whom the lung metastasis was their only residual cancer but surviving five years is not proof of cure. Patients with a solitary metastasis constitute 63% of the practice (19) and they live longer, however treated. But in most patients, the disease is present elsewhere as micrometastases, as is evident from the very many follow-up studies of lung metastasectomy from CRC. In the meta-analysis, 58% of 2,589 patients were dead before five years had elapsed after lung metastasectomy (19).
It is an old lesson that surgery is best reserved for curable cancer, and that systemic disease is best managed with systemic treatments (21). Despite claims that there are occasional long-term survivors, attribution of improved survival to lung metastasectomy was insecure in the absence of randomly assigned controls. The PulMiCC trial in 93 patients is sufficient to preclude the large benefit previously believed. The results are compatible with Åberg’s conjecture that case selection might be the explanation for apparently longer survival.
Our present position is that the previously believed difference in survival of >37% (that is an uplift from <5% to ~42%) is improbable based on the evidence gathered within PulMiCC. Its improbability is backed up by the supporting evidence in other RCTs which supply control survival for patients with CRC metastases amenable to local treatments but who did not have them resected or ablated. Those who would dismiss PulMiCC on the ground of the power calculation reveal an incomplete understanding of the term. It was coined to warn against planning studies which were inevitably going to be too small to exclude a small difference. Small differences require big numbers to reach statistical significance, whether for benefit or not. Once the data are in, they should be looked at, and understood and not discounted. It remains true that we cannot discount 5% or up to 10% difference in survival beyond five years, but there is very, very little likelihood that a larger trial would have confirmed the ~40% uplift in survival which has been believed, and that patients have been told.
There is a whole other way of thinking about lung metastases. The lung is the easiest part of the body to image. The metastases usually show up white, against a black background. All the observational evidence makes it improbable that it is the only site of disease, but it is the disease that can be measured and monitored. If the metastases remain static, or are growing very slowly, it would seem reasonable to hold off chemotherapy as there is something on which to gauge the activity of the cancer. Once you have taken the metastases out you are shooting in the dark again. The notion that taking the metastasis out is to give the patient a “chemo holiday” does not seem well founded. If neither metastasectomy or chemotherapy offers a useful gain for the patient, they should receive neither. What patients do not deserve is false hope, by taking over their last months and years with treatments that make them feel worse, with little realistic prospect of benefit. Giving false hope is to deceive the patient into believing things are other than they are, and that is not good medicine.
Acknowledgments
The authors acknowledge the contributions of all the Principle Investigators who contributed cases to the full PulMiCC study who were (in alphabetical order) Sion Barnard, Tim Batchelor, Aman Coonar, Brian Davidson, Joel Dunning, Jonathan Edwards, Simon Grummett, Jurjees Hassan, Ivo Hennig, Juliet King, Alan Kirk, Adrian Marchbank, Marcello Migliore, Kieran McManus, Babu Naidu, Apostolos Nakas, Michael Shackloth, Daniel Swinscow, Carol Tan, David Tsang, Telios Vakis, Mark Van Leuven, Yan Zheng and the many other trials staff in the centre and at the sites and not least the 512 patients.
Funding: Financial Disclosure: The PulMiCC trial was funded by Cancer Research UK funding Grant No. C7678/A11393
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marcello Migliore and Michel Gonzalez) for the series “VATS in Lung Metastasectomy” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/vats-2020-lm-08). The series “VATS in Lung Metastasectomy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stangl R, Altendorf-Hofmann A, Charnley RM, et al. Factors influencing the natural history of colorectal liver metastases. Lancet 1994;343:1405-10. [Crossref] [PubMed]
- Treasure T, Utley M, Hunt I. When professional opinion is not enough: surgical resection of pulmonary metastases. BMJ 2007;334:831-2. [Crossref] [PubMed]
- Poston G, Garden O, Primrose J, et al. Response to 'When professional opinion is not enough' BMJ 2007. BMJ [Internet]. 2007. Available online: https://www.bmj.com/content/334/7598/831/rapid-responses
- Mohamed F, Kallioinen M, Braun M, et al. Management of colorectal cancer metastases to the liver, lung or peritoneum suitable for curative intent: summary of NICE guidance. Br J Surg 2020;107:943-45. [Crossref] [PubMed]
- Grünhagen D, Jones RP, Treasure T, et al. The history of adoption of hepatic resection for metastatic colorectal cancer: 1984-95. Crit Rev Oncol Hematol 2013;86:222-31. [Crossref] [PubMed]
- Rosen CB, Nagorney DM, Taswell HF, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg 1992;216:493-504. [Crossref] [PubMed]
- Wei AC, Jarnagin WR. Questioning Why More Patients With Colorectal Liver Metastases Are Not Referred for Metastasectomy. JAMA Surg 2020;155:909-10. [Crossref] [PubMed]
- Fiorentino F, Hunt I, Teoh K, et al. Pulmonary metastasectomy in colorectal cancer: a systematic review and quantitative synthesis. J R Soc Med 2010;103:60-6. [Crossref] [PubMed]
- Åberg T, Malmberg KA, Nilsson B, et al. The effect of metastasectomy: fact or fiction? Ann Thorac Surg 1980;30:378-84. [Crossref] [PubMed]
- Fiorentino F, Vasilakis C, Treasure T. Clinical reports of pulmonary metastasectomy for colorectal cancer: a citation network analysis. Br J Cancer 2011;104:1085-97. [Crossref] [PubMed]
- MacRobert M, MacRoberts B. Problems of citation analysis. Scientometrics 1996;36:435-44. [Crossref]
- Van Raemdonck D, Friedel G. The European Society of Thoracic Surgeons Lung Metastasectomy Project. J Thorac Oncol 2010;5:S127-9. [Crossref] [PubMed]
- Treasure T, Fallowfield L, Lees B. Pulmonary metastasectomy in colorectal cancer: the PulMiCC trial. J Thorac Oncol 2010;5:S203-6. [Crossref] [PubMed]
- Thomford NR, Woolner L, Clagett O. The surgical treatment of metastatic tumours in the lung. J Thorac Cardiovasc Surg 1965;49:357-63. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Pfannschmidt J, Hoffmann H, Muley T, et al. Prognostic factors for survival after pulmonary resection of metastatic renal cell carcinoma. Ann Thorac Surg 2002;74:1653-7. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Haney JC, et al. Prognostic factors for recurrence after pulmonary resection of colorectal cancer metastases. Ann Thorac Surg 2009;87:1684-8. [Crossref] [PubMed]
- Pfannschmidt J, Hoffmann H, Dienemann H. Reported outcome factors for pulmonary resection in metastatic colorectal cancer. J Thorac Oncol 2010;5:S172-8. [Crossref] [PubMed]
- Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:572-9. [Crossref] [PubMed]
- Treasure T, Milošević M, Fiorentino F. Pulmonary metastasectomy and the use of molecular and radiological markers: is this a way to reduce unavailing surgery? Eur J Cardiothorac Surg 2014;45:417-8. [Crossref] [PubMed]
- Mukherjee S. The Emperor of All Maladies. New York: Scribner; 2010.
- Fenton H, Morris E. National variation in pulmonary metastasectomy for colorectal cancer; 2020.
- Mahase E. Covid-19: Demand for dexamethasone surges as RECOVERY trial publishes preprint. BMJ 2020;369:m2512. [Crossref] [PubMed]
- Migliore M, Milošević M, Lees B, et al. Finding the evidence for pulmonary metastasectomy in colorectal cancer: the PulMicc trial. Future Oncol 2015;11:15-8. [Crossref] [PubMed]
- Prasad V, Vandross A, Toomey C, et al. A decade of reversal: an analysis of 146 contradicted medical practices. Mayo Clin Proc 2013;88:790-8. [Crossref] [PubMed]
- Jawed I, Wilkerson J, Prasad V, et al. Colorectal Cancer Survival Gains and Novel Treatment Regimens: A Systematic Review and Analysis. JAMA Oncol 2015;1:787-95. [Crossref] [PubMed]
- Treasure T, Monson K, Fiorentino F, et al. The CEA Second-Look Trial: a randomised controlled trial of carcinoembryonic antigen prompted reoperation for recurrent colorectal cancer. BMJ Open 2014;4:e004385 [Crossref] [PubMed]
- Northover J, Houghton J, Lennon T. CEA to detect recurrence of colon cancer. JAMA 1994;272:31. [Crossref] [PubMed]
- Treasure T, Monson K, Fiorentino F, et al. Operating to remove recurrent colorectal cancer: have we got it right? BMJ 2014;348:g2085. [Crossref] [PubMed]
- Mokhles S, Macbeth F, Farewell V, et al. Meta-analysis of colorectal cancer follow-up after potentially curative resection. Br J Surg 2016;103:1259-68. [Crossref] [PubMed]
- Jeffery M, Hickey BE, Hider PN, et al. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev 2016;11:CD002200 [Crossref] [PubMed]
- Moertel CG, Schutt AJ, Go VL. Carcinoembryonic antigen test for recurrent colorectal carcinoma. Inadequacy for early detection. JAMA 1978;239:1065-6. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Treasure T, Baum M. An approach to randomization into surgical clinical trials. Br J Surg 2017;104:11-2. [Crossref] [PubMed]
- Treasure T, Farewell V, Macbeth F, et al. Pulmonary Metastasectomy versus Continued Active Monitoring in Colorectal Cancer (PulMiCC): a multicentre randomised clinical trial. Trials 2019;20:718. [Crossref] [PubMed]
- Milošević M, Edwards J, Tsang D, et al. Pulmonary Metastasectomy in Colorectal Cancer: updated analysis of 93 randomized patients - control survival is much better than previously assumed. Colorectal Dis 2020;22:1314-24. [Crossref] [PubMed]
- Ruers T, van Coevorden F, Punt CJ, et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J Natl Cancer Inst 2017;109:djx015 [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8. [Crossref] [PubMed]
- Handy JR, Bremner RM, Crocenzi TS, et al. Expert Consensus Document on Pulmonary Metastasectomy. Ann Thorac Surg 2019;107:631-49. [Crossref] [PubMed]
Cite this article as: Fiorentino F, Milošević M, Treasure T. What have we learned in the process of setting up and running the PulMiCC (Pulmonary Metastasectomy in Colorectal Cancer) randomised controlled trial? Video-assist Thorac Surg 2021;6:13.