Creation of multi-images for assistance in surgery for robotic segmentectomies
A few words on lung segmentectomy
While lobectomy is the gold standard treatment option for non-small cell lung carcinoma (NSCLC) today, lung segmentectomy is considered a valuable option in the treatment of early stage NSCLC, for lesions measuring less than 2 cm (T1a), notably in patients with multiple and important comorbidities, limited pulmonary functions, and geriatric groups.
Recently published comprehensive meta-analyses and systematic reviews showed not only the non-inferiority in survival between lobectomy vs. segmentectomy groups in the aforementioned groups of fragile patients, but also the lower rates of postoperative mortality and morbidities in segmentectomy than in lobectomy (1,2).
In regards to surgical techniques, segmentectomy by open thoracotomy was traditionally performed by dissecting and ligating the target segmental vessels and bronchus, then identifying the intersegmental plane by peeling the segmental vein. In the past, we also emphasized on the importance of palpating the lesion in order to secure the resection margins.
However, since video-assisted thoracoscopic approach was recommended for early stage NSCLC, we start noticing a changing trend from open to minimally invasive strategies, this limits the surgeon’s ability to palpate the parenchyma in a comprehensive manner (hence the need of new ways to visualize the target lesion), and witnessed a shift towards the use of mechanical staplers when it comes to intersegmental plane, even in open approaches.
This type of surgery can be very challenging to achieve due to the many anatomical variation that the surgeon could encounter, the difficulties in locating small size lesions and ground-glass opacities (GGOs), and finally securing enough margins around the lesion.
To overcome these difficulties, multi-images techniques were developed and reported in the medical literature.
In our review, we explore multi-images techniques in robot-assisted lung segmentectomy.
3D reconstruction of the pulmonary artery (PA), first reports
The segmental anatomy can indeed very complex and variable from one person to another, Gossot et al. explored some of the anatomical variations encountered by their team in a series of 390 full thoracoscopic segmentectomies (some during surgery, and others on 3D reconstructions preoperatively), the authors reach a conclusion that there is not a “standardized” anatomy, but only “variations” (3).
In order to overcome this, authors started using 3D reconstruction. Watanabe et al. were one of the first to report on the use of 3D-CT pulmonary angiography (3D-CTPA) to evaluate the PA branches in a series of 14 patients. They had a very high successful rate (98%) when the results were compared to intra-operative findings. In fact, it only missed 2 branches out of 86, both had very small diameter (less than 1.5 mm).
They suggest that this 3D navigation might enhance intra-operative security, and reduce surgery-related morbidity (4).
This report was followed by a larger series by Fukuhara et al. (5), they evaluated the role of 3D reconstruction of the PA in 49 patients undergoing VATS lobectomy for stage I lung cancer.
The model also has a remarkable success rate (95.2%) when results were compared to the intraoperative findings. Only 7 out of 146 branches were not detected, all of which were less than 2 mm in diameter. These reports highlight the accuracy of 3D-CTPA, and its possible role in enhancing intraoperative security as no case was converted to open thoracotomy for bleeding. However, they underline that care must be taken as small branches are not always detected.
Adding veins
After these reports on the efficacy of 3D-CT in studying the PA, Oizumi et al. (6) published a case report to evaluate the role of 3D simulation in case of thoracoscopic anatomical lung segmentectomy, where in contrast to open thoracotomy segmentectomy, surgeons cannot hold the lesion with their hands while dissecting the intersegmental plane; a technique that helps the surgeon secure good margins around the tumor.
In fact, they developed a 3D volume rendering of the PA and veins as well. Thus, enabling the operator to identify the intersegmental vein, thus the plane, and eventually securing the resection margin in minimally invasive segmentectomy.
The 3D reconstruction was done by the surgeon, and took around 10 minutes to be achieved.
Volonté et al. (7) followed by reporting on the usefulness of 3D reconstructions in the planning of a left S6 segmentectomy for an 0.6 mm lesion by small thoracotomy. They used the open-source version of Osirix© software (Pixmeo SARL, Switzerland) to reconstruct pulmonary vessels, those were transferred later to an iPad, which in turn was put in a plastic sterile cover and used on the operative field.
They highlighted the importance of such techniques not only in enhancing the safety of the procedure but also in teaching intrapulmonary anatomy for surgical residents and young surgeons.
More reports continued to be published combining 3D reconstructions of the vessels as well as the bronchi (8-10) for anatomical lobar and infra-lobar lung resections.
Robot-assisted surgery
In the recent years, robot-assisted surgery has gained increasing popularity in the field of thoracic surgery.
In fact, 3D high quality camera, tremor filtering, and the 7 degrees of movement help achieving precise dissections.
What is more interesting are the endless possibilities that this system offers by the integration of 3D models intra-operatively, and recently, the ability to switch to near infra-red (NIR) vision to visualize the indocyanine green (ICG), which can be used in multiple manners; the first is to visualize the intersegmental mental by injecting it intravenously after the target segmental vessels have been identified and stapled (11,12), or trans-bronchially as reported by Sekine et al. (13), the second is to use it as an endobronchial dye marker using radial endo-bronchial ultrasound (r-EBUS) (14) or electromagnetic navigational bronchoscopy (ENB) (15).
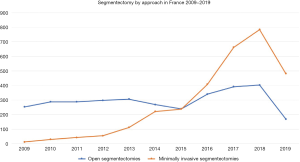
In France, we can clearly notice increasing numbers of segmentectomies in the past 10 years, as well as a shift towards video- and robot-assisted approaches to achieve this type of surgery (Figure 1). Data are extracted from Epithor database (Figure 1) (16).
Robot-assisted segmentectomy and dye marking
In 2014, Bolton et al. (17) published a retrospective review of their institutional database of patients who underwent ENB localization of lung nodules for robot-assisted resections between August 2012 and April 2013.
In 14 out of the 19 identified patients, a transbronchial biopsy was first done, followed by pleural dye marking using methylene blue dye. The remaining five patients had dye marking only.
Three out of the 14 biopsied patients had malignant diseases, thus surgical resection was carried out without the need for wedge resection. On the other hand, diagnostic segmentectomy was achieved in 4 patients, where the lesion was too deep for a wedge resection.
The remaining patients required a diagnostic wedge resection. The bronchoscopic procedure added a median time of 28 minutes to the overall time of the surgery. No cases were converted to open thoracotomy, and no complications were reported in relation to the placement of the dye marker. Success rate was 100%.
Krimsky et al. (18) also reported on ENB dye marking with methylene blue (n=11) and Indigo carmine dye (n=10) in 21 patients. However, the success rate was a bit lower (81%). There was no visible dye in three cases and the dye extravasated in one case, rendering the lesion impossible to visualize. No procedure related complications were reported, and all dye marking were followed up by video- or robot-assisted wedge resections.
In 2017, Abbas et al. (15) published their experience of 51 patients who underwent combined ENB dye marking of 54 nodules followed by infra-lobar anatomical resections. They used methylene blue, either alone or in addition to ICG and Isovue. In 2 cases, it was necessary to add a fiduciary. The success rate was that of 98.1%.
Minimally invasive surgery was performed in 49 of 51; by robotic-assisted thoracoscopic surgery (RATS) in 47 patients, and VATS in 2. Two patients had conversion to thoracotomy because of adhesions.
Geraci et al. (19) then published one of the largest series regarding ENB dye marking using ICG for robotic segmentectomy in 2019. A total of 245 patients who underwent planned robotic segmentectomy between January 2010 and October 2018 were included, of these, 93 had pleural dye marking using ICG by ENB, and all of them had received intravenous ICG to delaminate the resection margins, and the intersegmental plane. The lesions were successfully identified in 80 out of 93 cases, making a success rate of 86%.
In regards to pleural dye marking, they used 0.5 mL of 25 mg of ICG diluted in 10 mL of sterile water, this was rinsed by 0.5-mL saline, remaining at least 4 mm from the pleural surface. The remaining (9.5 mL) was injected intravenously after segmental artery ligation.
An R0 margins were obtained in 100% of the cases.
As we can conclude, the success rate of pleural dye marking is between 80% and 100% (15,17-20), and was in general superior to 95% in the majority of the series reported in the medical literature.
Meanwhile, despite its relatively good results, this technique presents some limitations including specific operative room preparations that ENB requires, and expensive disposable materials.
Another endobronchial approach was reported by Lachkar et al. (14) in 2018 using r-EBUS and virtual bronchoscopy for pleural dye marking. Between April 2016 and June 2017, all anticipated difficult minimally invasive infra-lobar resections of peripheral lesions were marked using this method. Twenty-five lesions (including 6 GGOs), were resected in 22 patients by VATS wedge resection (n=11), or RATS (n=11; 10 segmentectomies and 1 wedge resection).
The dye was visible on the pleural surface in 24 cases. Diagnosis and R0 margins were obtained in all. It’s noteworthy to say that the same operative precision was judged impossible by the operator in 21 cases if it was not for the pleural dye marking.
Building a multi-modal system for robot-assisted segmentectomies
Creating our multimodal approach to robot-assisted segmentectomy started back in 2014–2015, when we started a pilot study to evaluate 3D models usefulness in the operative planning, and enhancing efficiency and safety of robot-assisted segmentectomy (21). All included patients had contrast-enhanced chest CT scan with infra-millimetric slices (0.6 mm). A precise injection timing protocol was used to ensure visualization of the PA and pulmonary veins.
All images were anonymized, and sent electronically to a specialized private company (Visible PatientTM, Strasbourg, France) for 3D reconstruction (21,22).
After a few days, we received the resulting 3D images, those could be visualized either on an iPhone or an iPad using VP PLANNING app, or a personal computer. This can also be projected at the robotic console using Tile-Pro Mode© (Intuitive Surgical, California, USA).
During the aforementioned period, a total of 9 segmentectomies (1 right S1, 2 basal segmentectomies, 1 left S1+2, 3 right and left S6, 1 right S2 and 1 left S4+5) were performed with a pre-operative 3D model. No difference between the anatomy on the reconstructions and surgical dissection was found, that’s to say 100% of anatomical accuracy.
In 2018, we published our experience based on 114 robotic segmentectomies performed between January 2012 and October 2017. Back then, 55 of them were done between 2014 and 2017 using 3D reconstruction, little by little, we came into the conclusion that such 3D models are reliable, help the surgeon know beforehand the anatomic variation, predefine the resection strategy, and evaluate the resection margins beforehand, thus it improves the safety and the results of such difficult surgeries (22).
However, in that series, we missed one lesion that was too close to the intersegmental plane. For this reason, we decided to integrate pleural dye marking using radial endobronchial ultrasound and virtual bronchoscopy into our multimodal system, this is proved to be a useful way to extend resection margins, and help us perform extended segmentectomies.
The procedure starts with uploading the CT scan into a virtual bronchoscopy program (LungPoint® Planner), and after carefully studying the frontal, coronal and sagittal sections, the pulmonologist marks the target lesion, in order for the software to construct a route. This technique doesn’t provide real-time navigation, the operator memorizes, and can consult it on the computer at all times in case of doubt while achieving the endobronchial navigation.
The procedure consists of directing the bronchoscope following the predefined route, and upon arriving in the last accessible bronchus, the guide sheath with r-EBUS probe is inserted into the working channel, towards the target nodule till it reaches the subpleural space.
The probe is then removed and 1 mL of methylene blue (5 mg/1 mL) was injected and rinsed with 20 mL of air.
In fact, the purpose is to mark the nodule’s area, rather than the nodule itself, therefore helping the surgeon to quickly locate the resection zone. When NIR camera became available at the operative room with newer generation robots, we started doing double dye markings using 0.5 mL of methylene blue and 0.5 of ICG, thus the surgeon has full visibility on the lesion during normal as well as infra-red vision.
With the availability of NIR, we also started identifying the intersegmental plane using intravenous injections to ICG, after having identified and ligated the segmental artery and vein.
Thus, our multimodal systems in robot-assisted segmentectomies developed from 3D images integration to include the use of endobronchial dye marking and IV injection of ICG to delaminate the intersegmental plane (Figure 2).
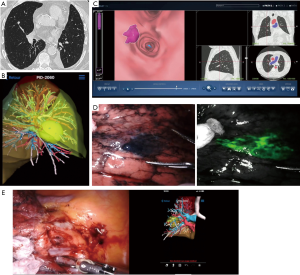
In 2019, we published our results of robot-assisted segmentectomies for lung metastases using this system (23), the series consisted of 168 patients operated of suspected lung metastases between 2012 and 2018. The majority (161 patients) were operated using minimally invasive techniques.
The number of patients operated by RATS was (n=55). Surgical resections were in the forms of single (n=5) or multiple wedges (n=3), segmentectomies (n=30), and lobectomies (n=17). 3D reconstruction was used in 11 segmentectomies. In 4 cases where the lesion was close to the intersegmental plane, we used pleural dye marking in addition to 3D reconstruction to secure the margins.
In 54 patients, resection margins were R0. The only incomplete resection occurred in the case of segmentectomy for renal cell carcinoma where the bronchial limits were R1.
Conclusions
The authors believe that lung segmentectomy is a key surgery to master by thoracic surgeons in the current era of technical innovation, ground glass nodules, and small lung carcinoma.
Endobronchial dye marking, 3D reconstructions, integration and the use of ICG in surgical robots can help the surgeon overcome many difficulties when it comes to this challenging surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Alper Toker) for the series “Robotic Segmentectomies” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats-20-21). The series “Robotic Segmentectomies” was commissioned by the editorial office without any funding or sponsorship. SL reports personal fees from Olympus and Fujifilm, outside the submitted work. JMB reports personal fees from Intuitive Surgery, Medtronic, and Johnson, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lim TY, Park S, Kang CH. A Meta-Analysis Comparing Lobectomy versus Segmentectomy in Stage I Non-Small Cell Lung Cancer. Korean J Thorac Cardiovasc Surg 2019;52:195-204. [Crossref] [PubMed]
- Bedetti B, Bertolaccini L, Rocco R, et al. Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 2017;9:1615-23. [Crossref] [PubMed]
- Gossot D, Seguin-Givelet A. Anatomical variations and pitfalls to know during thoracoscopic segmentectomies. J Thorac Dis 2018;10:S1134-44. [Crossref] [PubMed]
- Watanabe S, Arai K, Watanabe T, et al. Use of three-dimensional computed tomographic angiography of pulmonary vessels for lung resections. Ann Thorac Surg 2003;75:388-92. [Crossref] [PubMed]
- Fukuhara K, Akashi A, Nakane S, et al. Preoperative assessment of the pulmonary artery by three-dimensional computed tomography before video-assisted thoracic surgery lobectomy. Eur J Cardiothorac Surg 2008;34:875-7. [Crossref] [PubMed]
- Oizumi H, Endoh M, Takeda S, et al. Anatomical Lung Segmentectomy Simulated by Computed Tomographic Angiography. Ann Thorac Surg 2010;90:1382-3. [Crossref] [PubMed]
- Volonté F, Robert JH, Ratib O, et al. A lung segmentectomy performed with 3D reconstruction images available on the operating table with an iPad. Interact Cardiovasc Thorac Surg 2011;12:1066-8. [Crossref] [PubMed]
- 8Shimizu K, Nakazawa S, Nagashima T, et al. 3D-CT anatomy for VATS segmentectomy. J Vis Surg 2017;3:88.
- Eguchi T, Takasuna K, Kitazawa A, et al. Three-dimensional imaging navigation during a lung segmentectomy using an iPad. Eur J Cardiothorac Surg 2012;41:893-7. [Crossref] [PubMed]
- Ikeda N, Yoshimura A, Hagiwara M, et al. Three dimensional computed tomography lung modeling is useful in simulation and navigation of lung cancer surgery. Ann Thorac Cardiovasc Surg 2013;19:1-5. [Crossref] [PubMed]
- Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010;140:752-6. [Crossref] [PubMed]
- Tarumi S, Misaki N, Kasai Y, et al. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg 2014;46:112-5. [Crossref] [PubMed]
- Sekine Y, Ko E, Oishi H, et al. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012;143:1330-5. [Crossref] [PubMed]
- Lachkar S, Baste JM, Thiberville L, et al. Pleural Dye Marking Using Radial Endobronchial Ultrasound and Virtual Bronchoscopy before Sublobar Pulmonary Resection for Small Peripheral Nodules. Respiration 2018;95:354-61. [Crossref] [PubMed]
- Abbas A, Kadakia S, Ambur V, et al. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J Thorac Cardiovasc Surg 2017;153:1581-90. [Crossref] [PubMed]
- S.F.C.T.C.V. Société Française de Chirurgie Thoracique et Cardio-Vasculaire [Internet]. Société Française de Chirurgie Thoracique et Cardio-Vasculaire. [cited 2020 Feb 10]. Available online: https://www.sfctcv.org
- Bolton WD, Howe H, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5; discussion 475-6. [Crossref] [PubMed]
- Krimsky WS, Minnich DJ, Cattaneo SM, et al. Thoracoscopic detection of occult indeterminate pulmonary nodules using bronchoscopic pleural dye marking. J Community Hosp Intern Med Perspect 2014;4. [Crossref] [PubMed]
- Geraci TC, Ferrari-Light D, Kent A, et al. Technique, Outcomes With Navigational Bronchoscopy Using Indocyanine Green for Robotic Segmentectomy. Ann Thorac Surg 2019;108:363-9. [Crossref] [PubMed]
- Marino KA, Sullivan JL, Weksler B. Electromagnetic Navigation Bronchoscopy for Identifying Lung Nodules for Thoracoscopic Resection. Ann Thorac Surg 2016;102:454-7. [Crossref] [PubMed]
- Le Moal J, Peillon C, Dacher JN, et al. Three-dimensional computed tomography reconstruction for operative planning in robotic segmentectomy: a pilot study. J Thorac Dis 2018;10:196-201. [Crossref] [PubMed]
- Baste JM, Soldea V, Lachkar S, et al. Development of a precision multimodal surgical navigation system for lung robotic segmentectomy. J Thorac Dis. 2018;10:S1195-204. [Crossref] [PubMed]
- Sarsam M, Peillon C, Baste JM. Robotic pulmonary metastasectomy using precision multimodal surgical navigation. J Vis Surg 2019;5:53. [Crossref]
Cite this article as: Sarsam M, Lachkar S, Baste JM. Creation of multi-images for assistance in surgery for robotic segmentectomies. Video-assist Thorac Surg 2021;6:5.