
Oncological clearance of uniportal vats for early stage non-small cell lung cancer
Introduction
The advent of uniportal video-assisted thoracic surgery (uVATS) lobectomy in 2010 polarised the surgical community. Although it promises the least access trauma of any minimally invasive thoracic surgical approach to date, critics argue that oncological clearance may be compromised by inadequate exposure and suboptimal nodal staging. The same doubts once cast on multiportal VATS (mVATS) for resectable non-small cell lung cancer (NSCLC) in decades past have been revisited with uVATS. In this article, the state of evidence regarding oncological clearance of uVATS is examined and directions for the future are explored.
Uniportal VATS—access trauma and oncological outcomes
VATS is the term encompassing thoracic operations performed via small incisions with purely endoscopic visualisation of intrathoracic viscera. In contrast, open lobectomy is performed under direct vision through a large thoracotomy. The muscle splitting, rib cutting and spreading required to gain access to the pleural cavity by the open approach often result in chronic pain, shoulder dysfunction and disability (1). The access trauma incurred and the resultant systemic inflammatory response syndrome (SIRS) is usually severe. VATS not only reduces pain and disability, but also could limit the magnitude of SIRS. This is hypothesised to reduce postoperative disturbances in cellular and humoral immunity and prevent an environment which could favour tumour micrometastases (2-4). Thus, reducing the incision length may potentially not only reduce pain, but also reduce immune dysfunction and possibly risks of disease recurrence. To evolve from multiportal to uniportal VATS could be an approach to benefit from such access trauma reduction. There is early data to suggest that uniportal VATS may be associated with an attenuated post-operative immunochemokine response compared with multiportal, however more studies are need to confirm this and to investigate its clinical significance (3).
Evolution of uniportal VATS
In the minds of many, a reduction in port or ports usually equate to limited access and maneuverability of the surgical instruments. However, since the emergence of uniportal VATS for major lung resection almost a decade ago, we have seen a rapid development in instruments and surgical technique (5). There is no doubt that evolution from triportal to uniportal requires the surgeon to adapt to new planes of visualisation and instrumentation (6,7). An understanding of the stepwise geometric basis of evolution of VATS approaches can help dispel misconceptions that visualisation and instrumentation is restricted when the converse may hold true (8,9).
In the triportal approach, the thoracoscope and rigid straight instruments are placed in a baseball diamond configuration corresponding to a rhombus, with the thoracoscope at home base, the hilum or target at second base, and instruments at first and third base. The three instruments are aimed towards the same vanishing point (10). The posterior port corresponding to first base is used for lung retraction and introduction of tissue staplers. The main issue with this approach is that instruments passed from the posterior port may interfere with the thoracoscope. In attempts to avoid the posterior instrument, the first assistant may drive the thoracoscope to an optical plane different from the instrumentation plane, presenting a perspective which hinders hand-eye coordination.
In the biportal approach, the posterior port is eliminated, and the anterior port is a utility mini-thoracotomy incision with passage of multiple instruments. This has a number of important implications for the surgeon. First, the plane of instrumentation is translated from a horizontal, caudocranial perspective to a vertical, anteroposterior perspective. Second, curved instruments may be required in order to reach the whole thoracic cavity and to minimise fencing. Third, crossing manipulations are required to bring about effective traction-countertraction, and these maneuvers require transferring the effective fulcrum of instrumentation inside the chest cavity. Finally, the plane of instrumentation now runs perpendicular to the plane of visualization, hence could lead to more significant hand-eye inconsistency which may be difficult to overcome.
In the uniportal approach, the inferior thoracoscopy port is eliminated, and the anterior incision must now also encompass the thoracoscope. The plane of visualization is also translated to a vertical anteroposterior perspective, same as for the plane of instrumentation. The axes of thoracoscopic view and instrumentations thus become parallel. This simulates direct vision in a thoracotomy, and hand-eye inconsistency is minimized (8,9). It is interesting to note that it may be for this reason numerous centres have adopted the uniportal VATS approach directly from open surgery without transitioning through multiport VATS (11).
Metrics of oncological clearance in lung cancer surgery
Anatomical complete resection and mediastinal lymph node dissection that allow accurate pathological staging offers the best chance of cure for medically operable patients with resectable early-stage non-small cell lung cancer. The oncological clearance of lung cancer surgery very much hinges on the completeness of mediastinal lymph node staging. A point of contention for oncological clearance of any minimally invasive approach is whether differences in instrumentation and surgical technique hinders adequate nodal harvesting and thus the opportunity for nodal upstaging. With a reported incidence of nodal upstaging varying from 9% to 24%, a substantial portion of patients with pathological node-positive disease may benefit from adjuvant therapy if they are not understaged by suboptimal lymph node harvesting (12).
There is no consensus on the definition of a complete mediastinal lymph node staging. The 2019 National Comprehensive Cancer Network (NCCN) guidelines recommend either sampling at least three N2 stations or complete lymphadenectomy. Although mediastinal lymphadenectomy can theoretically identify more skip metastatic lesions and occult lymph node metastases, whether it leads to improved survival remains controversial (13).
In a 2017 analysis of large-scale Chinese and US multi-institutional databases, a greater number of examined lymph nodes positively correlated with more stage migration and better overall survival in patients with node-negative disease. The cutoff was calculated to be sixteen examined nodes (14). To implement this metric in practice, surgeons should adhere to a stringent protocol for intraoperative nomenclature and enumeration of lymph nodes, as fragmentation may over-estimate the number of nodes harvested (15). This cutoff may allow for an additional metric for a more confident declaration of pathological node-negative disease, and could give the surgeon and the patient greater reassurance.
Is uVATS oncologically equivalent to mVATS?
To ascertain the state of the evidence regarding oncological efficacy of uVATS, the authors searched Medline and EMBASE using the strategy listed in the International Society for Minimally Invasive Cardiac Surgery (ISMICS) expert consensus statement on “Optimal Approach to Lobectomy for Non-Small Cell Lung Cancer: Systematic Review and Meta-Analysis” (16).
From January 2000 to October 2019, twenty-three articles comparing mVATS and uVATS were identified. Two studies were excluded from the current discussion because they did not report any oncological outcomes. The twenty-one remaining studies, involving 3,737 mVATS patients and 2,165 uVATS patients in total, were all retrospective in nature (10,17-36). Propensity score matching was performed in ten studies. Except for one conference abstract, the other articles were published in peer-reviewed journals. Characteristics of the included studies are listed in Table 1.
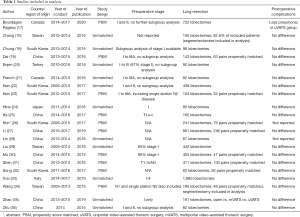
Full table
Sixteen studies reported the rate of early postoperative complications. However, there was significant heterogeneity in how complications were defined and classified. Overall, there was no significant difference in complications between the two groups. Bourdages-Pageau et al. reported less pneumonia in the uVATS group than mVATS, but the cause of this observation is unclear.
Eighteen studies compared the number of dissected lymph nodes harvested. Radiological nodal staging was often only partially reported or not reported at all. Routine lymphadenectomy was performed in thirteen studies, lymph node sampling in two studies, and in three studies the strategy is unclear. None of the articles defined the boundaries of lymph node stations or completeness of lymphadenectomy. Only one study reported the number of lymph nodes harvested from N1 stations and N2 stations separately. All the studies showed that uVATS is non-inferior to mVATS in terms of number of lymph nodes stations sampled as well as number of lymph nodes harvested. Interestingly, the propensity matched study by Song et al. showed that the uVATS group had more lymph nodes harvested; however, the reason for this observation is unclear (32). Four articles reported the rate of pathological upstaging and no significant difference was found. Details on oncological outcomes of these eighteen studies are listed in Table 2.
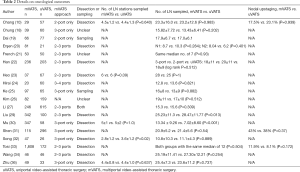
Full table
Only two retrospective studies reported data on short- to mid-term survival. Han et al. from South Korea reported the results of 439 VATS lobectomies for stage I and II disease from 2006 to 2015, during the group’s transition from triportal to biportal and then to uniportal. The three-year overall survival was 87.3% for the triportal group (median follow-up of 75.7 months), compared with 93.7% for biportal (median follow-up of 56.5 months), and 93.2% for uniportal (median follow-up 27.5 months). There was no difference in both overall survival and disease-free survival between the three groups (22). Zhao et al. from China retrospectively reviewed results of 191 lobectomies performed on patients with T1a and T1b disease from 2013 to 2015. There was no difference in three-year overall survival between the thoracotomy group, multiportal group and uniportal group (P=0.327) (35). However, the exact percentage of surviving patients at three years in each group was not reported.
In 2014, our institution reported one of the first large-scale case series of uVATS lobectomies involving one-hundred thirty NSCLC patients. Despite the high prevalence of pulmonary tuberculosis in Asia, our experience is that the uVATS approach was not hindered by the presence of anthracotic hilar lymph nodes or dense pleural adhesions, as evidenced by a low conversion rate of 5.3% (2). The two-year overall disease-free survival rate was 96% for stage I disease and 83% for stage II and above, which was in line with these two studies.
Finally, the elephant in the room must be addressed. None of the studies explained the rationale behind the choice of VATS approach. The choice was largely a matter of surgeon’s discretion. One article frankly stated that “the selection criteria between single-port and triple-ports were not special or different” (36). Even with propensity score matching, the risk of selection bias was deemed to be high because only three out of ten studies reported the parameters incorporated in propensity score calculation.
The effect of learning curve
Uniportal VATS is difficult to master. Dedicated and focused training is required at high-volume centres with close mentoring by experienced surgeons. Furthermore, surgeon and institutional experience has been shown to affect oncological clearance. An audit of five-hundred consecutive VATS lobectomies at New York-Presbyterian Hospital showed that significantly more lymph nodes were harvested in the latter half of the cohort. Further analysis of the learning curve of an individual surgeon in VATS lymphadenectomy showed that a plateau in the number of lymph nodes harvested was reached after the initial fifty cases (37). Gonzalez et al. analysed their initial three years of experience with VATS lobectomies at Coruna, during the group’s transition from triportal to biportal. Two-hundred cases from 2007 to 2010 were divided into three cohorts by year and with increased experience, each year saw an improvement in nodal harvesting (38).
Although mVATS is the approach adopted in both studies, the results can be extrapolated to uVATS. Zhongshan University analysed the learning curve of the first one-hundred and twenty uVATS lobectomies performed by a group of experienced mVATS surgeons from 2013 to 2014 (39). The skin-to-skin time reached a plateau after the first thirty cases. Furthermore, there were significantly more conversions and unsuccessful attempts at passing the stapler in the first quartile of the cohort. Our own experience is that trainees should gain experience with biportal instrumentation first before transitioning to uniportal. In the event of technical difficulties, conversion to biportal approach can in most cases ensure safe and expedient conduct of the operation, by allowing more instruments to be introduced into the operating field, reduce instrument fencing and increase stapling angles.
Conclusions
Although there are no limitations intrinsic to the uniportal approach that compromises oncological efficacy, the use of uVATS for lung cancer remains controversial. Retrospective comparative studies suggest that oncological clearance of uVATS is equivalent to mVATS in terms of nodal staging and early mid-term survival, but their results should be interpreted with caution because of selection bias and lack of long-term follow-up. We echo Dr. Gonzalez-Rivas’ call for more high-quality research in this arena, in order to ascertain the true value of uVATS in the armamentarium of modern minimally invasive thoracic surgery (40).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mingyon Mun) for the series “Oncological Clearance of VATS Lobectomy for Clinical N0 Non-small Cell Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2020.04.02). The series “Oncological Clearance of VATS Lobectomy for Clinical N0 Non-small Cell Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. CSHN serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Jun 2019 TO May 2021. CSHN reports personal fees from Johnson and Johnson, USA, personal fees from Medtronic USA, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li WL, Lee LM, Lee TW, et al. The impact of thoracic surgical access on early shoulder function: video-assisted thoracic surgery versus posterolateral thoracotomy. Eur J Cardiothorac Surg 2003;23:390-6. [Crossref] [PubMed]
- Ng CSH, Kim HK, Wong RHL, et al. Single-Port Video-Assisted Thoracoscopic Major Lung Resections: Experience with 150 Consecutive Cases. Thorac Cardiovasc Surg 2016;64:348-53. [Crossref] [PubMed]
- Yu PSY, Lau RWH, Wan IYP, et al. Uniportal Video Assisted Thoracic Surgery For Major Lung Resection Is Associated With Less Immunochemokine Disturbances. Int Soc Minim Invasive Cardiothorac Surg Annu Sci Meet 29 May - 1 Jun 2019, New York, USA Abstr 19-A-262-ISMICS.
- Ng CSH, Wan S, Hui CWC, et al. Video-assisted thoracic surgery lobectomy for lung cancer is associated with less immunochemokine disturbances than thoracotomy. Eur J Cardiothorac Surg 2007;31:83-7. [Crossref] [PubMed]
- Ng CSH, Lau KKW, Gonzalez-Rivas D, et al. Evolution in surgical approach and techniques for lung cancer. Thorax 2013;68:681. [Crossref] [PubMed]
- Ng CSH, Wong RHL, Lau RWH, et al. Minimizing chest wall trauma in single-port video-assisted thoracic surgery. J Thorac Cardiovasc Surg 2014;147:1095-6. [Crossref] [PubMed]
- Ng CSH, Wong RHL, Lau RWH, et al. Single port video-assisted thoracic surgery: Advancing scope technology. Eur J Cardiothorac Surg 2015;47:751. [Crossref] [PubMed]
- Bertolaccini L, Rocco G, Pardolesi A, et al. The Geometric and Ergonomic Appeal of Uniportal Video-Assisted Thoracic Surgery. Thorac Surg Clin 2017;27:331-8. [Crossref] [PubMed]
- Bertolaccini L, Rocco G, Viti A, Terzi A. Geometrical characteristics of uniportal VATS. J Thorac Dis 2013;5:S214-6. [PubMed]
- Chang JM, Kam KH, Yen YT, et al. From biportal to uniportal video-assisted thoracoscopic anatomical lung resection: A single-institute experience. Medicine (Baltimore) 2016;95:e5097 [Crossref] [PubMed]
- Anile M, Diso D, Mantovani S, et al. Uniportal video assisted thoracoscopic lobectomy: Going directly from open surgery to a single port approach. J Thorac Dis 2014;6:S641-3. [PubMed]
- Martin JT, Durbin EB, Chen L, et al. Nodal upstaging during lung cancer resection is associated with surgical approach. Ann Thorac Surg 2016;101:238-44; discussion 244-5. [Crossref] [PubMed]
- Zhong W, Yang X, Bai J, et al. Complete mediastinal lymphadenectomy: the core component of the multidisciplinary therapy in resectable non-small cell lung cancer. Eur J Cardiothorac Surg 2008;34:187-95. [Crossref] [PubMed]
- Liang W, He J, Shen Y, et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-small-cell lung cancer: A population study of the US SEER database and a Chinese multi-institutional registry. J Clin Oncol 2017;35:1162-70. [Crossref] [PubMed]
- Handy JR, Costas K, Nisco S, et al. Regarding American College of Surgeons Commission on Cancer Non-Small Cell Lung Cancer Quality of Care Measure 10RLN. Ann Thorac Surg 2016;102:1040-1. [Crossref] [PubMed]
- Ng CSH, Macdonald JK, Gilbert S, et al. Optimal Approach to Lobectomy for Non-Small Cell Lung Cancer: Systemic Review and Meta-Analysis. Innovations (Phila) 2019;14:90-116. [Crossref] [PubMed]
- Bourdages-Pageau E, Vieira A, Lacasse Y, et al. Outcomes of Uniportal vs Multiportal Video-Assisted Thoracoscopic Lobectomy. Semin Thorac Cardiovasc Surg 2020;32:145-51. [Crossref] [PubMed]
- Chung JH, Choi YS, Cho JH, et al. Uniportal video-assisted thoracoscopic lobectomy: An alternative to conventional thoracoscopic lobectomy in lung cancer surgery? Interact Cardiovasc Thorac Surg 2015;20:813-9. [Crossref] [PubMed]
- Dai F, Meng S, Mei L, et al. Single-port video-assisted thoracic surgery in the treatment of non-small cell lung cancer: A propensity-matched comparative analysis. J Thorac Dis 2016;8:2872-8. [Crossref] [PubMed]
- Erşen E, Kiliç B, Kara HV, et al. Uniportal versus multiport video-assisted thoracoscopic surgery for anatomical lung resections: A glance at a dilemma. Wideochir Inne Tech Maloinwazyjne 2018;13:215-20. [Crossref] [PubMed]
- French DG, Thompson C, Gilbert S. Transition from multiple port to single port video-assisted thoracoscopic anatomic pulmonary resection: Early experience and comparison of perioperative outcomes. Ann Cardiothorac Surg 2016;5:92-9. [Crossref] [PubMed]
- Han KN, Kim HK, Choi YH. Midterm outcomes of single port thoracoscopic surgery for major pulmonary resection. PLoS One 2017;12:e0186857 [Crossref] [PubMed]
- Heo W, Kang DK, Min HK, et al. Feasibility and safety of single-port video-assisted thoracic surgery for primary lung cancer. Korean J Thorac Cardiovasc Surg 2017;50:190-6. [Crossref] [PubMed]
- Hirai K, Takeuchi S, Usuda J. Single-incision thoracoscopic surgery and conventional video-assisted thoracoscopic surgery: A retrospective comparative study of perioperative clinical outcomes. Eur J Cardiothorac Surg 2016;49:i37-41. [PubMed]
- Ke H, Liu Y, Zhou X, et al. Anterior fissureless uniport vs. posterior intra-fissure triple-port thoracoscopic right upper lobectomy: A propensity-matched study. J Thorac Dis 2017;9:3866-74. [Crossref] [PubMed]
- Kim HK, Han KN, Choi YH. PUB025 Comparison of Surgical Outcomes between Multiport and Single Port Thoracoscopic Lobectomy for Lung Cancer: Propensity Score Matched Analysis. J Thorac Oncol 2017;12:S1461-2.
- Li J, Qiu B, Scarci M, et al. Uniportal video-assisted thoracic surgery could reduce postoperative thorax drainage for lung cancer patients. Thorac Cancer 2019;10:1334-9. [PubMed]
- Lin F, Zhang C, Zhang Q, et al. Uniportal video-assisted thoracoscopic lobectomy: An alternative surgical method for pulmonary carcinoma. Pak J Med Sci 2016;32:1283-5. [PubMed]
- Liu CC, Shih CS, Pennarun N, et al. Transition from a multiport technique to a single-port technique for lung cancer surgery: Is lymph node dissection inferior using the single-port technique? Eur J Cardiothorac Surg 2016;49:i64-72. [PubMed]
- Mu JW, Gao SG, Xue Q, et al. A matched comparison study of uniportal versus triportal thoracoscopic lobectomy and sublobectomy for early-stage nonsmall cell lung cancer. Chin Med J (Engl) 2015;128:2731-5. [Crossref] [PubMed]
- Shen Y, Wang H, Feng M, et al. Single- versus multiple-port thoracoscopic lobectomy for lung cancer: A propensity-matched study. Eur J Cardiothorac Surg 2016;49:i48-53. [PubMed]
- Song KS, Park CK, Kim JB. Efficacy of single-port video-assisted thoracoscopic surgery lobectomy compared with triple-port VATS by propensity score matching. Korean J Thorac Cardiovasc Surg 2017;50:339-45. [Crossref] [PubMed]
- Tosi D, Nosotti M, Bonitta G, et al. Uniportal and three-portal video-assisted thoracic surgery lobectomy: analysis of the Italian video-assisted thoracic surgery group database. Interact Cardiovasc Thorac Surg 2019;29:714-21. [Crossref] [PubMed]
- Wang BY, Liu CY, Hsu PK, et al. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: A propensity-matched analysis. Ann Surg 2015;261:793-9. [Crossref] [PubMed]
- Zhao R, Shi Z, Cheng S. Uniport video assisted thoracoscopic surgery (U- VATS) exhibits increased feasibility, non-inferior tolerance, and equal efficiency compared with multiport VATS and open thoracotomy in the elderly non-small cell lung cancer patients at early. Medicine (Baltimore) 2019;98:e16137 [Crossref] [PubMed]
- Zhu Y, Liang M, Wu W, et al. Preliminary results of single-port versus triple-port complete thoracoscopic lobectomy for non-small cell lung cancer. Ann Transl Med 2015;3:92. [PubMed]
- Lee PC, Kamel M, Nasar A, et al. Lobectomy for non-small cell lung cancer by video-assisted thoracic surgery: Effects of cumulative institutional experience on adequacy of lymphadenectomy. Ann Thorac Surg 2016;101:1116-22. [Crossref] [PubMed]
- Gonzalez D, de la Torre M, Paradela M, et al. Video-assisted thoracic surgery lobectomy: 3-year initial experience with 200 cases. Eur J Cardiothorac Surg 2011;40:e21-8. [Crossref] [PubMed]
- Liu X, Chen X, Shen Y, et al. Learning curve for uniportal video-assisted thoracoscopic surgery lobectomy—results from 120 consecutive patients. J Thorac Dis 2018;10:5100-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Damico TA, Jiang G, et al. Uniportal video-assisted thoracic surgery: A call for better evidence, not just more evidence. Eur J Cardiothorac Surg 2016;50:416-7. [Crossref] [PubMed]
Cite this article as: Lim K, Chan JWY, Yu PSY, Wan IYP, Lau RWH, Ng CSH. Oncological clearance of uniportal vats for early stage non-small cell lung cancer. Video-assist Thorac Surg 2020;5:25.


