
Socioeconomic, rural, and insurance-based inequities in robotic lung cancer resections
Introduction
Poor, rural, and underinsured patients with lung cancer experience significant treatment-based disparities (1-5). For instance, lung cancer patients who live in neighborhoods with lower socioeconomic status (SES) and rural areas are less likely to undergo surgery or to receive chemotherapy or radiotherapy (1,6,7). Similarly, lung cancer patients who are uninsured and who have Medicaid have lower odds of receiving curative operations (5). These disparities contribute to well-established inferior outcomes in underserved lung cancer populations (1-5). Furthermore, as underserved groups typically have less access to cutting-edge treatments, the benefits associated with these often costly medical and technological advances are only experienced by a select, socioeconomically advanced few, further widening treatment disparity gaps (8). The use of robotic-assisted surgery appears to be no exception.
The first robotic lobectomies were reported in 2003, and their use has increased rapidly over the past decade (9,10). This increase is in part attributed to the advantages of robotic thoracic surgery over video-assisted thoracoscopic surgery (VATS) (11,12). Studies have shown significant benefits of robotic surgical technology, including reduced blood loss, shorter length of hospital stay, increased lymph node retrieval rates, fewer surgical complications, and reduced strain on the surgeon (13-25). Unfortunately, wide adoption is limited by high capital and running costs of robotic instruments (15). In the robotic prostate cancer literature, racial minorities and patients with lower SES in non-metropolitan locations have been found to have less access to robot-assisted radical prostatectomy (RARP)-performing hospitals (14,26). In addition, Medicaid patients are less likely to receive RARP than privately insured patients (14,26).
Other fields, including surgical and gynecological oncology, have found similar disparities in the use of robotic surgeries based on SES, proximity to academic centers, and insurance status (13,27-30), suggesting that the same may contribute to disparities in lung cancer. However, there is a paucity of information on barriers to robotic surgery access specifically for patients with lung cancer. This study aims to elucidate the sociodemographic factors that contribute to reduced access to robotic lung cancer surgery more than 15 years since its first implementation.
Methods
Data Sources
The National Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality (AHRQ), is a database of annual hospital inpatient discharges, representing 97% of the US population, and the largest all-payer inpatient care database in the US (31,32). We sought to characterize the use of robotic lobectomy for lung cancer versus that of open or VATS lobectomy. Specifically, we sought information related to patient or hospital characteristics potentially affecting the application of these three approaches for lobectomy.
NIS databases for the years 2010 to 2014 were queried for adult patients (age ≥18) with a diagnosis of lung cancer. We included patients with malignant neoplasm of the lung and airway with diagnosis codes 162.0–162.9. From this group, a subset of patients receiving lobectomy were selected. The NIS data sets use diagnosis and procedure codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Principal or secondary procedures coded as open lobectomy (ICD-9-CM 32.4 or 32.49) without thoracoscopic or robotic procedure modifiers were categorized as open, while VATS lobectomy (ICD-9-CM 32.41) alone or with an open lobectomy code was categorized as VATS. Principal or secondary procedures that included codes for both lobectomy and a robotic procedure (ICD-9-CM 17.4) were categorized as robotic.
Patient characteristic variables included patient age, gender, race (White, Black, Hispanic, Native American, Asian/Pacific Island, and other), primary expected payer [Medicare, Medicaid, private insurance, uninsured (self-pay; no charge), or other], median household income for each patient’s ZIP code (by quartile), admission type (non-elective, elective), length of stay (LOS), discharge disposition (routine, transfer to another hospital, and others), transfer status (to another acute care or other type of health facility), and vital status. Patient comorbidity was quantified using the Deyo modification of the Charlson Comorbidity Index, excluding subscales for cancer and presence of metastatic disease (33-35). Hospital characteristics included size (small, medium, large, based on number of beds) and location-by-teaching status (rural, urban, or urban-teaching). The NIS hospital size variable was relative to hospital location (rural/urban) because rural hospitals tend to have fewer beds compared with hospitals in urban centers.
Statistical analysis
Each year of NIS data was trend-weighted so that results would be representative of the population of US inpatients for that year. As instructed by HCUP, updated trend weights were applied to NIS data for the years 2010 and 2011 to produce frequency estimates comparable to the new state-by-hospital sampling methodology initiated with the 2012 data set.
For tables of descriptive information, continuous variables are reported as mean and standard deviation (SD; LOS) or median and interquartile range (age); categorical variables are reported as frequencies and percentages. Bivariate statistical analyses were conducted to compare differences in demographic, clinical, and hospital characteristics by lobectomy procedure type (open versus robotic and VATS versus robotic). For continuous variables, independent sample student’s t-tests were applied (age, LOS); either Pearson’s chi-squared or Fisher’s exact tests were applied to categorical variables.
Multivariable logistic regression was applied to determine predictors of (a) open versus robotic lobectomy or (b) VATS versus robotic lobectomy.
All analyses were performed using IBM SPSS Statistics version 25.0 (IBM Corp., Armonk, NY) and SAS version 9.4 (SAS Institute Inc., Cary, NC). All tests of significance were two-sided, and the value of alpha for statistical significance was P<0.05.
Results
Patient and operative characteristics
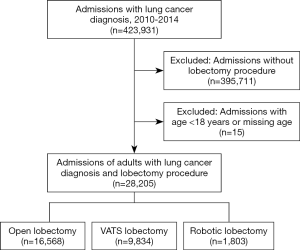
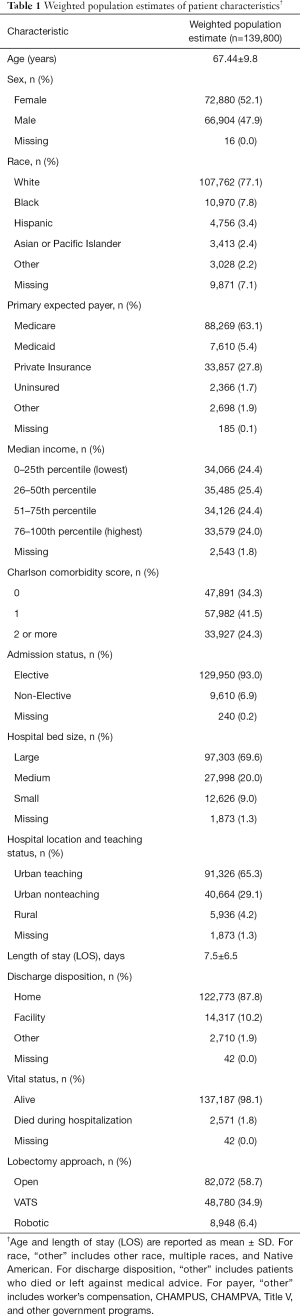
Within the NIS from 2010 to 2014, there were 28,205 admissions of adults who were diagnosed with lung cancer and underwent lobectomy procedures, categorized as open [16,568 (58.7%)], VATS [9,834 (34.9%)], or robotic [1,803 (6.4%)] (Figure 1). The trend-weighted population estimate was 139,800 patients; their demographic and hospital information are shown in Table 1.
Full table
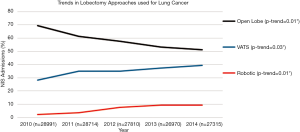
From 2010 to 2014, the proportion of open lobectomies performed decreased significantly from 69.4% to 51.2% (P value for trend 0.01), whereas the proportion of VATS lobectomies performed increased from 28.2 to 39.4% (P value for trend 0.03) and the proportion of robotic lobectomies performed increased from 2.4 to 9.4% (P value for trend 0.01) (Figure 2).
Characteristics associated with robotic lobectomy in univariable analysis
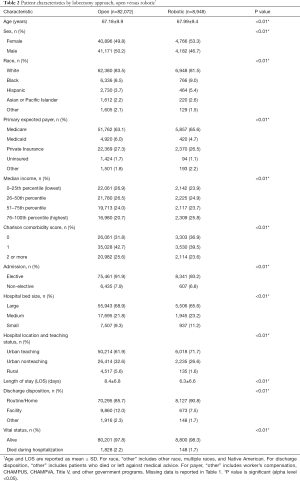
Univariate analysis comparing patients who underwent robotic versus open lobectomy is shown in Table 2. Compared to patients who underwent open lobectomy, patients who underwent robotic lobectomy were more likely to be women (53.3% vs. 49.8%, P<0.01) and have a higher median income. They were also more likely to have a lower Charlson comorbidity score and an elective admission (93.2% vs. 91.9%, P<0.01) and to be in an urban teaching hospital (71.7% vs. 61.9%, P<0.01). Patients who underwent robotic lobectomy had a shorter mean LOS (6.3% vs. 8.4 days, P<0.01), were more likely to be discharged to home (90.8% vs. 85.7%, P<0.01), and had a lower inpatient mortality rate (1.7% vs. 2.2%, P<0.01).
Full table
The results of univariate analyses comparing patients who underwent robotic versus VATS lobectomy are shown in Table 3. Compared to patients who underwent VATS lobectomy, patients who underwent robotic lobectomy were less likely to be women (53.3% vs. 55.8%, P<0.01) and have a lower median income. They were also less likely to be in a large hospital (65.6% vs. 74.1%, P<0.01) and had a higher inpatient mortality rate (1.7% vs. 1.2%, P=0.001). The difference in LOS between these groups was statistically but not clinically significant (6.3 vs. 6.2 days, P<0.01).
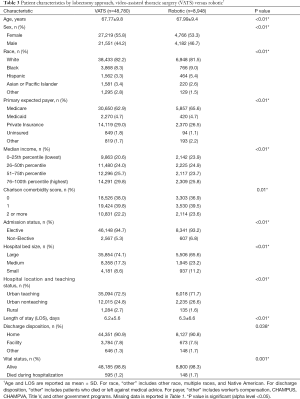
Full table
Characteristics associated with robotic lobectomy in multivariable analysis
Multivariable analyses comparing patients who underwent robotic versus open and robotic versus VATS lobectomy are shown in Table 4. Age, sex, Charlson comorbidity score, admission status, and hospital bed size were included in the models. Low-income patients were less likely to undergo robotic versus open lobectomy (AOR =0.78, P<0.01), but were more likely to undergo robotic versus VATS lobectomy (AOR =1.31, P<0.01). Compared to patients in urban teaching hospitals, patients in rural hospitals were much less likely to undergo robotic versus open (AOR =0.28, P<0.01) or VATS (AOR =0.64, P<0.01) lobectomy. Compared to patients with Medicare, patients with Medicaid were less likely to undergo robotic compared to open (AOR =0.80, P<0.01) or VATS (AOR =0.88, P=0.049) lobectomy. Similarly, uninsured patients were also less likely to undergo robotic versus open (AOR =0.62, P<0.01) or VATS (AOR =0.50, P<0.01) lobectomy.
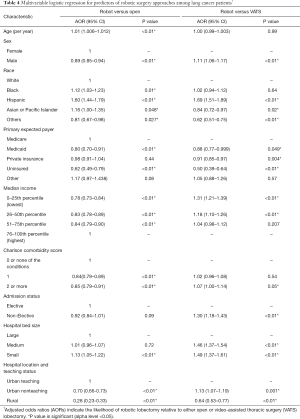
Full table
Compared to White patients, Black patients were more likely to undergo robotic versus open lobectomy (AOR =1.12, P=0.01), but were no more likely to have robotic versus VATS lobectomy (AOR =1.02, P=0.64). Hispanic patients were more likely to have robotic versus open (AOR =1.60, P<0.01) or VATS (AOR =1.69, P<001) lobectomy.
Discussion
This study leveraged the NIS database to identify sociodemographic factors that were associated with the use of robotic lung cancer resection. We found that neighborhood-level income, rural location of the hospital, and insurance status significantly impact whether a patient with lung cancer is treated with robotic resection rather than open or VATS lobectomy. These factors have been shown to impact access to robotic resections in other cancers (14,29,36) but had not previously been evaluated in lung cancer patients.
Socioeconomic status
In this study, low neighborhood-level income decreased the odds of a lung cancer patient undergoing robotic versus open lobectomy. This finding is consistent with other studies that have shown an association between neighborhood-level SES and access to oncologic treatments, particularly robotic-assisted procedures (14,29,36). Patients who live in deprived neighborhoods may have lower access to or live further from hospitals that have the additional resources necessary to provide costly robotic surgery. Thus, the finding that patients living in poorer neighborhoods are less likely to obtain robotic versus open lung cancer resections was not surprising.
What was surprising and difficult to explain was our finding that low neighborhood-level income increased the odds of robotic surgery versus VATS resection. Many studies have found that robotic resections are more costly than thoracoscopic ones (37,38). The increased cost burden of robotic surgery is therefore expected to reduce its use in areas with low neighborhood-level income. Our findings suggest that, despite the high costs associated with robotic resections, low neighborhood-level income favors this approach over other minimally invasive lobectomy techniques. This finding has not been previously shown and may shed light on the fact that the impact of SES on outcomes can be quite difficult to interpret. Neighborhood-level and individual-level SES can impact outcomes independently (39) and cannot be assumed to be identical. Therefore, the finding that those in areas of low neighborhood-level income had higher rates of robotic versus VATS lobectomy should not be interpreted to mean that low individual income or poverty leads to more robotic surgery versus thoracoscopic resections. Furthermore, neighborhood-level income is only one component of neighborhood SES, and absence of other variables like neighborhood education and neighborhood deprivation data, due to limitations of the NIS database, restricts our ability to comprehensively understand how neighborhood SES impacts robotic lung cancer surgery access. For now, we can only state that patients living in areas with the lowest median neighborhood-level incomes had higher rates of open lobectomies, but lower rates of VATS resections, compared to robotic lobectomies. Certainly, we will need to evaluate this finding in larger studies with more granular, comprehensive SES data.
Rurality
Living in rural areas is associated with geographic isolation and less access to primary and specialty care (40,41) and is therefore a well-known risk factor associated with lower oncologic treatment access (1). Because of increased poverty and under-resourced hospitals in rural locations, advanced technologies are less prevalent and have delayed implementation. Our findings that patients in rural hospitals were much less likely to undergo robotic versus open or VATS lobectomy was consistent with most other studies that have evaluated the impact of rurality on treatment access (1,6,41).
Insurance
Patients with no or public insurance have consistently been found to be at higher risk for worse outcomes and lower treatment rates than those with private insurance (4,5). In line with these findings, as well as many other robotic cancer resection studies, (14,29,30) we found that robotic lobectomies were less common in those with no insurance or with Medicaid. This finding has important implications in the context of the expansion of Medicaid eligibility under the Affordable Care Act, as growth of the number of Medicaid enrollees may lead to worse disparities due to robotic lobectomy access.
Race/ethnicity
We were surprised that Black patients had higher rates of robotic lobectomies vs. open and Hispanic patients had higher rates of robotic lobectomies versus open or VATS lobectomies, indicating that there is no racial disparity in robotic lung cancer resection access. Whereas previous studies have found that race impacts urologic robotic cancer resection rates (14,26), others focused on robotic gastrointestinal cancer resections, including one using the NIS database, also found that race did not influence access to robotic or minimally invasive procedures (29,42).
Limitations of this study include the fact that the NIS is an administrative inpatient database populated by ICD-9 codes. NIS underwent significant methodological changes that impacted the numbers of discharges in the dataset. These changes complicate the comparison of discharges and hospital bed sizes between 2010–2011 and 2012–2014 and necessitate the use of discharge weights when analyzing historical NIS files as we have done here.
As noted earlier, this study was also limited by the fact that the NIS does not collect individual-level social determinants, so an evaluation of the impact of these individual determinants on robotic lung cancer surgery access was not possible. And although NIS tracks neighborhood-level median income, it does not gather other neighborhood factors such as neighborhood education or deprivation. Therefore, it is not possible to comprehensively evaluate the impact of neighborhood SES on robotic lung cancer surgery access. Furthermore, the NIS records whether hospitals are in rural locations but not whether the patients themselves reside in rural locations. It is certainly conceivable that a patient and the hospital that they choose/have access to are not located in the same type of place. Lastly, detailed information about tumor characteristics, which may impact surgical approach decisions, are unavailable.
To conclude, we have found that neighborhood-level income, rural hospital location, and insurance status impact whether a patient obtains robotic lung cancer resections via lobectomy. These findings shine a light on sociodemographic factors that may limit access to technology that could significantly improve outcomes. Rural, poor, and underinsured patients with lung cancer fundamentally have worse outcomes and have been previously found to be less likely to receive any treatment, including surgery and chemotherapy, than those who are less socioeconomically deprived (7). Our current findings reveal that these groups also have less access to robotic surgeries. As the use of robotic surgery continues to grow, our understanding of disparities with respect to access will become more important and currently deserves greater attention. If these issues are not addressed, a major disadvantage of the robotic lung cancer surgery approach will be disparate access to the underserved. This study contributes to the literature by characterizing important sociodemographic influences on robotic lung cancer resections and raises questions that may be addressed by future public health policies. It is essential to understand barriers that may result in disparities in access to robotic lung cancer resections if we are to eliminate them.
Acknowledgments
Special thanks to Kerin Higa, PhD for her assistance in reviewing and editing of the manuscript.
Funding: This research was supported by the National Institutes of Health (2 K12 CA001727).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Natalie S. Lui and Sean C. Wightman) for the series “Robotic Surgery for Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2020.02.01). The series “Robotic Surgery for Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. NSL served as the unpaid Guest Editor of the series. NSL reports non-financial support from Intuitive Surgical, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and individual informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Atkins GT, Kim T, Munson J. Residence in rural areas of the united states and lung cancer mortality. disease incidence, treatment disparities and stage-specific survival. Ann Am Thorac Soc 2017;14:403-11. [Crossref] [PubMed]
- Erhunmwunsee L, Joshi MB, Conlon DH, et al. Neighborhood-level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer 2012;118:5117-23. [Crossref] [PubMed]
- Lynge E. Unemployment and cancer: a literature review. IARC Sci Publ 1997;343-51. [PubMed]
- Ellis L, Canchola AJ, Spiegel D, et al. Trends in cancer survival by health insurance status in california from 1997 to 2014. JAMA Oncol 2018;4:317-23. [Crossref] [PubMed]
- Stokes SM, Wakeam E, Swords DS, et al. Impact of insurance status on receipt of definitive surgical therapy and posttreatment outcomes in early stage lung cancer. Surgery 2018;164:1287-93. [Crossref] [PubMed]
- Johnson AM, Hines RB, Johnson JA 3rd, et al. Treatment and survival disparities in lung cancer: the effect of social environment and place of residence. Lung Cancer 2014;83:401-7. [Crossref] [PubMed]
- Forrest LF, Adams J, Wareham H, et al. Socioeconomic inequalities in lung cancer treatment: systematic review and meta-analysis. PLoS Med 2013;10:e1001376 [Crossref] [PubMed]
- Nishi A, Milner DA Jr, Giovannucci EL, et al. Integration of molecular pathology, epidemiology and social science for global precision medicine. Expert Rev Mol Diagn 2016;16:11-23. [Crossref] [PubMed]
- Linsky P, Wei B. Robotic lobectomy. J Vis Surg 2017;3:132. [Crossref] [PubMed]
- Ahn MJ, Won HH, Lee J, et al. The 18p11.22 locus is associated with never smoker non-small cell lung cancer susceptibility in Korean populations. Hum Genet 2012;131:365-72. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42. [Crossref] [PubMed]
- Ramadan OI, Wei B, Cerfolio RJ. Robotic surgery for lung resections-total port approach: advantages and disadvantages. J Vis Surg 2017;3:22. [Crossref] [PubMed]
- Berkley BS GE. Disparities in Robotic Surgery for Gastrointestinal Cancers. J Robotics Autom 2017;1:8-9.
- Kim J, ElRayes W, Wilson F, et al. Disparities in the receipt of robot-assisted radical prostatectomy: between-hospital and within-hospital analysis using 2009-2011 California inpatient data. BMJ Open 2015;5:e007409 [Crossref] [PubMed]
- Novellis P, Alloisio M, Vanni E, et al. Robotic lung cancer surgery: review of experience and costs. J Vis Surg 2017;3:39. [Crossref] [PubMed]
- Brooks P. Robotic-assisted thoracic surgery for early-stage lung cancer: a review. AORN J 2015;102:40-9. [Crossref] [PubMed]
- Cerfolio RJ, Ghanim AF, Dylewski M, et al. The long-term survival of robotic lobectomy for non–small cell lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Emmert A, Straube C, Buentzel J, et al. Robotic versus thoracoscopic lung resection: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7633 [Crossref] [PubMed]
- Kim MP. Robotic lobectomy leads to excellent survival in lung cancer patients. J Thorac Dis 2018;10:S3184-5. [Crossref] [PubMed]
- Liang H, Liang W, Zhao L, et al. Robotic versus video-assisted lobectomy/segmentectomy for lung cancer: a meta-analysis. Ann Surg 2018;268:254-9. [Crossref] [PubMed]
- Singh H, Modi HN, Ranjan S, et al. Robotic surgery improves technical performance and enhances prefrontal activation during high temporal demand. Ann Biomed Eng 2018;46:1621-36. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Wei B, Eldaif SM, Cerfolio RJ. Robotic lung resection for non–small cell lung cancer. Surg Oncol Clin N Am 2016;25:515-31. [Crossref] [PubMed]
- Park BJ. Robotic lobectomy for non-small cell lung cancer: long-term oncologic results. Thorac Surg Clin 2014;24:157-62. vi. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term survival based on the surgical approach to lobectomy for clinical stage I nonsmall cell lung cancer: comparison of robotic, video-assisted thoracic surgery, and thoracotomy lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Kim SP, Boorjian SA, Shah ND, et al. Disparities in access to hospitals with robotic surgery for patients with prostate cancer undergoing radical prostatectomy. J Urol 2013;189:514-20. [Crossref] [PubMed]
- Fader AN, Weise RM, Sinno AK, et al. Utilization of minimally invasive surgery in endometrial cancer care: a quality and cost disparity. Obstet Gynecol 2016;127:91-100. [Crossref] [PubMed]
- Forde GK, Chang J, Liu F, et al. Disparities in access to minimally invasive surgery for women undergoing hysterectomy in california. Obstet Gynecol 2014;123:28S. [Crossref] [PubMed]
- Gabriel E, Thirunavukarasu P, Al-Sukhni E, et al. National disparities in minimally invasive surgery for rectal cancer. Surg Endosc 2016;30:1060-7. [Crossref] [PubMed]
- Probst CP, Aquina CT, Kelly KN, et al. Location matters: factors associated with access to robotic surgery for rectal cancer. J Am Coll Surg 2014;219:e147 [Crossref]
- HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP) (2010-2011). Agency for Healthcare Research and Quality, Rockville, MD. Available online: https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed November 18 2019.
- HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP) (2012-2014). Agency for Healthcare Research and Quality, Rockville, MD. Available online: https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed November 18 2019.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9. [Crossref] [PubMed]
- Robins JM. Data, design, and background knowledge in etiologic inference. Epidemiology 2001;12:313-20. [Crossref] [PubMed]
- Turner M, Adam MA, Sun Z, et al. Insurance status, not race, is associated with use of minimally invasive surgical approach for rectal cancer. Ann Surg 2017;265:774-81. [Crossref] [PubMed]
- Basil S. Nasir ASB, Douglas J. Minnich, Ben Wei, Robert J. Cerfolio. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg 2014;98:203-9. [Crossref]
- Swanson SJ, Miller DL, McKenna RJ, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: Results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [Crossref] [PubMed]
- Wu CC, Chang CM, Hsu TW, et al. The effect of individual and neighborhood socioeconomic status on esophageal cancer survival in working-age patients in Taiwan. Medicine (Baltimore) 2016;95:e4140 [Crossref] [PubMed]
- Hing E, Hsiao CJ. State variability in supply of office-based primary care providers: United States, 2012. NCHS Data Brief 2014;1-8. [PubMed]
- Seervai S. Practicing medicine in rural american: listening to primary care physicians. Commonwealth Fund 2019.
- Alnasser M, Schneider EB, Gearhart SL, et al. National disparities in laparoscopic colorectal procedures for colon cancer. Surg Endosc 2014;28:49-57. [Crossref] [PubMed]
Cite this article as: Erhunmwunsee L, Bhandari P, Sosa E, Sur M, Ituarte PHG, Lui NS. Socioeconomic, rural, and insurance-based inequities in robotic lung cancer resections. Video-assist Thorac Surg 2020;5:13.


