Impact of the number of resected lymph nodes during multi-portal VATS lobectomy for clinical N0 non-small cell lung cancer
Introduction
Video-assisted thoracoscopic surgery (VATS) lobectomy with systematic mediastinal lymph node evaluation has been adopted worldwide for lung cancer surgery due to improved early outcomes—lesser pain, a shorter hospital stay, fewer complications, and a higher compliance to adjuvant chemotherapy compared to thoracotomy—without compromising oncologic outcomes (1-6). Meanwhile, accurate mediastinal lymph nodes evaluation plays a pivotal role by providing precise staging and a therapeutic purpose of loco-regional control, which contribute to improved long-term survival after surgery. Current guideline recommends that N1 and N2 node resection should be a routine component of lung cancer resections—a minimum of three N1 and three N2 stations should be sampled or completely dissected based on the International Association for the Study of Lung Cancer nodal map (7,8). There have been some studies suggesting the removal of a minimum of 10 lymph nodes, however, this number ranges up to 16 lymph nodes depending on the study (9-11). Despite of the guideline which suggests to evaluate and remove lymph nodes, the adherence to the guideline is quite poor (12,13).
The number of resected lymph nodes can be a surrogate for the quality of VATS lobectomy in lung cancer surgery. However, the number of resected lymph nodes is influenced by the patient, surgeon’s preference, and tumor factors. Therefore, the efficacy of lymph node evaluation in VATS varies. Several studies based on a large national cohort showed that VATS was associated with a lower detection rate for unsuspected nodal metastasis compared to thoracotomy (14,15). On the contrary, some articles showed that there were no differences in the efficacy of lymph node dissection and related long-term survival according to the surgical approach (16,17). In particular, the type of facility and penetration of surgical approaches were associated with the number of resected lymph node and station, which may have contributed to the difference in proficiency of VATS surgery for lung cancer (9,14). Furthermore, inadequate lymph node evaluation during surgical resection is still quite common, irrespective of surgical approaches (13). With the given heterogeneous results from previous literature, the adequacy of lymphadenectomy based on the number of resected lymph nodes in VATS for patients with non-small cell lung cancer (NSCLC) has been questioned.
Hence, we aimed to reveal oncological long-term results following VATS lobectomy and mediastinal lymph dissection for clinical N0 NSCLC, focusing on the quality of lymph node dissection.
Methods
Study population
From April of 2005 to June of 2013, a total of 2,502 patients underwent curative surgical resection of NSCLC at Seoul National University Hospital. Among them, multi-portal VATS lobectomy was performed in 1,055 patients (42.2%). We identified 974 eligible patients during the study period following the inclusion criteria: (I) who underwent VATS lobectomy for lung cancer with clinical N0 disease, (II) pathologically confirmed as NSCLC. We excluded 81patients based on the following criteria: (I) small cell histology, (II) wedge resection, segmentectomy and pneumonectomy, (III) intentional thoracotomy, and (IV) who were treated with neoadjuvant chemo or radiotherapy. VATS has been adopted for lung cancer since April 2005 in our institute. Currently, we predominantly perform VATS in over 80% of all lung cancer surgeries. All surgical candidates for lung cancer were evaluated with a chest CT scan and whole body FDG PET. In our institution, at least three N2 stations (2R, 4R, 7, 9 for right side and 4L, 5, 6, 7, 9 for left side), hilar, interlobar and peribronchial lymph nodes were completely dissected during the surgical resection except for anthracofibrosis of lymph nodes were technically impossible to be resected completely and mediastinal lymph node dissection was intentionally avoided. In addition, we preferred an en-bloc resection of N1 lymph nodes during lobectomy to avoid fragmentation. Complete mediastinal lymph node dissection was defined when at least three N2 stations were examined. This study was approved and an informed consent was waived by the Institutional Review Board in our institute.
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 (IBM, Inc., Armonk, NY, USA). In the univariable analysis, categorical variables were compared using a χ2 test. Continuous variables were compared using the Student’s t-test. Log-rank test was used in analyzing the survival rate. The relationship between the long-term survival and the number of removed lymph nodes was analyzed using Cox-regression proportional model. A P value of <0.05 was considered statistically significant.
Results
Clinical characteristics
Patients and operative details
A total of 974 patients were enrolled (Table 1). The median follow up duration was 73 months. The mean age was 62.7 years. The majority of the patients were clinical stage I (n=911, 93.5%) and adenocarcinoma (n=807, 82.9%) was the dominant histology. The postoperative morbidity rate was 17.8% and the 30-day mortality was 0.3%. The median hospital stay was 5 days. Conversion to thoracotomy were identified in 21 patients (2.2%).
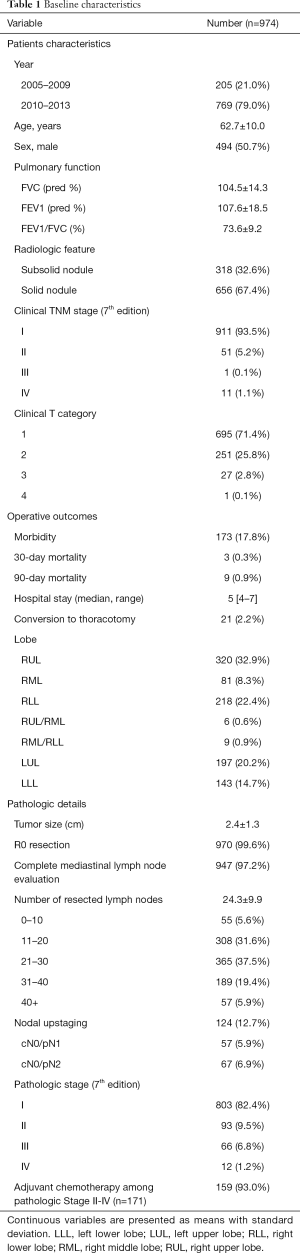
Full table
Pathologic outcomes
The mean tumor sized was 2.4 cm. R0 resection was achieved in 970 patients (99.6%). Mean number of resected lymph nodes were 24. Three or more mediastinal lymph nodal stations were evaluated in 947 patients (97.2%). More than 10 lymph nodes in 919 patients (94.4%). Nodal upstaging was found in 124 patients (12.7%). Unsuspected N1 and N2 were identified in 57 patients (5.9%) and 67 patients (6.9%), respectively. Adjuvant treatment was employed in 159 patients (93.0%) among pathologic stage II-IV.
Long-term prognosis and the number of resected lymph nodes
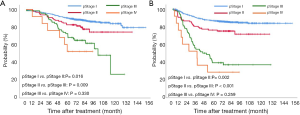
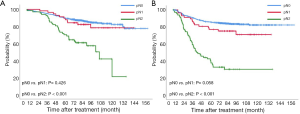
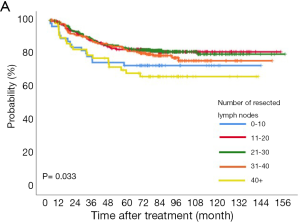
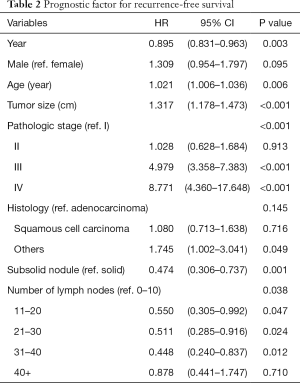
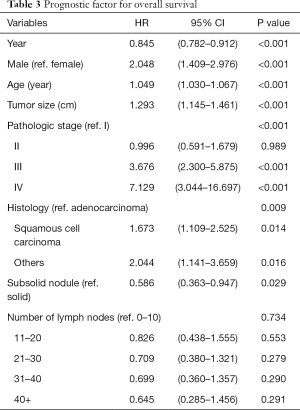
The 5-year overall and recurrence-free survival rates were 86.3% and 80.4%, respectively. The 10-year overall and recurrence-free survival rates were 78.3% and 77.9%, respectively. Recurrences developed in 196 patients (20.1%) and the most common pattern was distant metastasis (n=125, 12.8%). Loco-regional recurrences were found in 71 (7.3%) patients including 20 (2.1%) cases of dissected area, 27 (2.8%) cases of non-dissected area, 24 (2.5%) cases who had isolated pleural seeding. Post-recurrence survival duration was 33 months. There were clear differences in the overall survival (P<0.001) and recurrence-free survival (P<0.001) according to pathologic stages (Figure 1). Pathologic N2 showed the worst prognosis. Interestingly, pathologic N1 group had a similar overall and recurrence-free survival rate with those of pathologic N0 group (Figure 2). There was a significant difference in the recurrence-free survival according to the number lymph nodes harvested by 10-increment (P=0.033). Kaplan-Meier curves according to the number of resected lymph node by 11–20, 21–30, and 31–40 groups were similar; however, 0–10 and 40+ group tended to show the worse prognosis compared to the other groups (Figure 3). A multivariable Cox-regression analysis revealed that the number of removed lymph nodes by 10-increment was a significant prognostic factor for the recurrence-free survival after adjusting for year, age, tumor size, pathologic stage, histology, and radiologic features (Table 2). For the number of resected lymph nodes, the number of resected lymph nodes 11–20, 21–30, 31–40 groups were associated with improved recurrence-free survival unlike the group in which only 0–10 lymph nodes were removed. However, a significant survival benefit was no longer observed when more than 40 lymph nodes were removed. The number of resected lymph nodes was not a significant prognostic factor for overall survival (Table 3).
Full table
Full table
Discussion
We demonstrated long-term oncological outcomes of VATS lobectomy and mediastinal lymph node dissection for clinical N0 NSCLC based on a single institution’s initial experience. Therefore, we were able to provide long-term survival rates and detailed recurrence patterns in depth with the sufficient follow up duration. In our institution, over 90% of the patient underwent complete mediastinal lymph node dissection and satisfactory overall and recurrence-free survival were achieved. The present study revealed a significant association between the number of resected lymph nodes and long-term survival. Furthermore, harvesting lymph nodes ranging 11 to 40 was associated with improved recurrence-free survival. There was no incremental improvement in the prognostic value after 40+ lymph nodes resection.
Systematic lymph node dissection allows accurate staging and subsequent improved survival. The AJCC TNM staging system for lung cancer uses the nodal stations based on IASLC nodal map, rather than the number of lymph nodes unlike gastrointestinal, breast, and bladder cancers (18). Therefore, there is no definite guideline for the number of examined lymph nodes during operation. The current guideline recommends at least six nodal stations to be examined three from N1 and three from N2 stations (7,8,19). Several studies suggest that at least 10 to 16 lymph nodes should be removed to improve long-term outcomes (9-11,20). Meanwhile, nodal upstaging is a frequently used surrogate for the completeness of node evaluation in the field of cancer surgeries. A recent large scale cohort study based on the National Cancer Database reported that incidence of unsuspected pN1 and pN2 in cN0 disease after lobectomy were 6.7% (8,915/132,604) and 3.9% (5,192/132,604), respectively (21). Our study showed similar nodal upstaging rates with previous literature, irrespective of surgical approach (14,15,21,22). Association between nodal upstaging and the survival advantage has been contradictory so far. Interestingly, although our data showed a good separation according to pathologic stages, the unsuspected pathologic N1 group showed a comparable long-term survival rate with those of pathologic N0 group. These results may imply that complete dissection of metastatic hilar and peribronchial lymph nodes may have been performed during surgical resection and may also have led to a therapeutic effect.
The quality of lymph node dissection is different according to the facility and surgical approach (9,13-15). The reason for a low incidence of nodal upstaging of VATS is associated with the preferential use of VATS or thoracotomy. In addition, peripheral tumors tend to have lower nodal metastasis than central tumors. This may have influenced the selection for VATS for its surgical approach which may have results showing a discrepancy (23,24). Some surgeons argue that a fissureless technique to avoid air leakage may be related to low number of lymph nodes retrieved (25). The impact of the advanced surgical approach on the efficacy of lymph node assessment is not clear. The development of instrumentation such as robotic and 3D videoscope may allow delicate dissection and substantial high yield of lymph node (22,25). Wilson et al. reported that robotic lobectomy achieved similar nodal upstaging with that of thoracotomy, which was significantly higher than that of VATS (22). On the contrary, a large retrospective study showed no association between robotic and VATS for nodal upstaging and long-term survival (17). However, the overall number of harvested lymph nodes, around 10, from each surgical approach is quite low compared to our data. Moreover, the difference according to surgical approach disappeared in the academic/research facility (14,15). Therefore, we should interpret with caution due to the heterogeneity of studies regarding lymph node dissection.
A precise counting of resected lymph nodes is obscured by fragmentation during the operation, especially in VATS. Different institutions, surgeons, and pathologists affect the assessment of the number and completeness of lymph nodes examined. Although four surgeons performed VATS lobectomy for lung cancer during the study period, institutional policy with regards to preoperative evaluation for lung cancer and preferential use of mediastinal lymph node dissection rather than sampling were not quite different among the surgeons. Upstaging following surgical resection depends on the accuracy of clinical staging as well. During the study period, preoperative evaluation modality and technology had evolved, particularly, the routine use of PET-CT and EBUS-TBNA (26,27). Therefore, we included the year of surgery as a covariate to adjust those temporal changes.
Despite several limitations related to a retrospective study in a single center, we demonstrated satisfactory long-term survival after VATS lobectomy for N0 NSCLC and the oncologic clearance by VATS lobectomy focused on the number of resected lymph nodes. We concluded that the number of removed lymph nodes was closely related with recurrence-free survival after VATS lobectomy. Therefore, we claim that proper lymph node dissection should be performed in VATS lobectomy despite its difficulties.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mingyon Mun) for the series “Oncological Clearance of VATS Lobectomy for Clinical N0 Non-small Cell Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2020.02.03). The series “Oncological Clearance of VATS Lobectomy for Clinical N0 Non-small Cell Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Seoul National University Hospital Clinical Research Institute with informed consent not required (IRB No. 1911-139-1080).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang CJ, Kumar A, Klapper JA, et al. A National Analysis of Long-term Survival Following Thoracoscopic Versus Open Lobectomy for Stage I Non-small-cell Lung Cancer. Ann Surg 2019;269:163-71. [Crossref] [PubMed]
- Nicastri DG, Wisnivesky JP, Litle VR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg 2008;135:642-7. [Crossref] [PubMed]
- Su S, Scott WJ, Allen MS, et al. Patterns of survival and recurrence after surgical treatment of early stage non-small cell lung carcinoma in the ACOSOG Z0030 (ALLIANCE) trial. J Thorac Cardiovasc Surg 2014;147:747-52: Discussion 752-3.
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951-60; discussion 960-1. [Crossref] [PubMed]
- Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg 2008;85:231-5; discussion 235-6. [Crossref] [PubMed]
- Cao C, Zhu ZH, Yan TD, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: a propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg 2013;44:849-54. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 7.2019). Accessed November, 2019.
- AJCC Cancer Staging manual, 8th ed. New York: Springer; 2017.
- Krantz SB, Lutfi W, Kuchta K, et al. Improved Lymph Node Staging in Early-Stage Lung Cancer in the National Cancer Database. Ann Thorac Surg 2017;104:1805-14. [Crossref] [PubMed]
- Liang W, He J, Shen Y, et al. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol 2017;35:1162-70. [Crossref] [PubMed]
- American College of Surgeons. CoC measure for quality of cancer care. Accessed November, 2019.
- Odell DD, Feinglass J, Engelhardt K, et al. Evaluation of adherence to the Commission on Cancer lung cancer quality measures. J Thorac Cardiovasc Surg 2019;157:1219-35. [Crossref] [PubMed]
- Edwards T, Balata H, Elshafi M, et al. Adequacy of Intraoperative Nodal Staging during Surgical Resection of NSCLC: Influencing Factors and Its Relationship to Survival. J Thorac Oncol 2017;12:1845-50. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 353. [Crossref] [PubMed]
- Medbery RL, Gillespie TW, Liu Y, et al. Nodal Upstaging Is More Common with Thoracotomy than with VATS During Lobectomy for Early-Stage Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2016;11:222-33. [Crossref] [PubMed]
- D'Amico TA, Niland J, Mamet R, et al. Efficacy of mediastinal lymph node dissection during lobectomy for lung cancer by thoracoscopy and thoracotomy. Ann Thorac Surg 2011;92:226-31; discussion 231-2. [Crossref] [PubMed]
- Hennon MW, DeGraaff LH, Groman A, et al. The association of nodal upstaging with surgical approach and its impact on long-term survival after resection of non-small-cell lung cancer. Eur J Cardiothorac Surg 2020;57:888-95. [PubMed]
- Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84.
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Wen YS, Xi KX, Xi KX, et al. The number of resected lymph nodes is associated with the long-term survival outcome in patients with T2 N0 non-small cell lung cancer. Cancer Manag Res 2018;10:6869-77. [Crossref] [PubMed]
- Razi SS, Nguyen D, Villamizar N. Lobectomy Does Not Confer Survival Advantage Over Segmentectomy For Non-Small Cell Lung Cancer with Unsuspected Nodal Disease. J Thorac Cardiovasc Surg 2019; [Epub ahead of print]. [PubMed]
- Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6; discussion 1906-7.
- Lee PC, Port JL, Korst RJ, et al. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:177-81. [Crossref] [PubMed]
- Decaluwe H, Stanzi A, Dooms C, et al. Central tumour location should be considered when comparing N1 upstaging between thoracoscopic and open surgery for clinical stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2016;50:110-7. [Crossref] [PubMed]
- Toker A, Ozyurtkan MO, Kaba E. Nodal upstaging: effects of instrumentation and three-dimensional view in clinical stage I lung cancer. J Vis Surg 2017;3:76. [Crossref] [PubMed]
- Park HK, Jeon K, Koh WJ, et al. Occult nodal metastasis in patients with non-small cell lung cancer at clinical stage IA by PET/CT. Respirology 2010;15:1179-84. [Crossref] [PubMed]
- Vial MR, O'Connell OJ, Grosu HB, et al. Diagnostic performance of endobronchial ultrasound-guided mediastinal lymph node sampling in early stage non-small cell lung cancer: A prospective study. Respirology 2018;23:76-81. [Crossref] [PubMed]
Cite this article as: Park S, Kang CH, Lee HJ, Park IK, Kim YT. Impact of the number of resected lymph nodes during multi-portal VATS lobectomy for clinical N0 non-small cell lung cancer. Video-assist Thorac Surg 2020;5:12.