
Transitioning from VATS to robotic lobectomy
Introduction
Advances in surgical techniques have heralded significant growth in the realm of robotic assisted technologies. iData Research demonstrates that over 693,000 robotic assisted procedures were performed in 2017 in the United States with continued growth associated with newer platforms offering market competition (1). Most recent estimates value the market for surgical robotic systems at over $2.4 billion worldwide with the US accounting for 73% of surgical volume (2). Intuitive Surgical’s da Vinci robotic system remains the dominant platform in the total robotic-assisted surgery system market. Their market lead can be attributed to their strong showing in the minimally invasive surgery market overall for all specialties. By the end of 2017, the company shipped 5,770 robot systems; after accounting for trade-ins and returns, 4,409 platforms were installed globally including 2,862 (65%) in the United States. The estimated annual procedure volume increased from 136,000 in 2008 to 877,000 in 2017 (2). Within cardiothoracic surgery, robotic surgery was initially developed with minimally invasive cardiac procedures in mind, however non-cardiac thoracic cases soon became the most frequent use of robotics within the specialty (3). With its adoption, there has been a gradual but steady decline in the prevalence of open lobectomies being performed in the US. From 2011 to 2015, the number of open lobectomies experienced an absolute decline of 11.5% during which time the numbers of minimally invasive lobectomies including both video-assisted thoracoscopic (VATS) and robotic-assisted thoracoscopic (RATS) saw an increase of 1.5% and 10% respectively. Currently, the prevalence of robotic lobectomy is estimated at 18% of all lobectomies (4).
Benefits of robotic approach to lobectomy
There are several reported advantages associated with use of the robotic approach to lobectomy which are attributed to manipulating the robotic arms and instruments controlled by computer-assisted systems. The result of which is reduction of surgeon tremor and intuitive translation of the natural wristed movement of the surgeon's hands into the surgical field. While innumerable studies have reported on the benefits of minimally-invasive approaches (predominantly VATS) compared to traditional open thoracotomy in the domains of post-operative pain, length of stay, and peri-operative morbidity, studies directly comparing VATS and RATS for lobectomy have been fewer in number and inconsistent in establishing a clear advantage of one approach versus the other (5) even during the learning curve (6). Several single institutional studies have shown reduced pain, decreased length of stay, and higher yield of lymph node dissection associated with the robotic approach (3,6). However, in a metanalysis of 14 retrospective cohort studies there was no significant difference between VATS and RATS lobectomy with respect to conversion to open thoracotomy, number of dissected lymph nodes, hospital length of stay, operative time, length of chest tube drainage, incidence of prolonged air leak, and morbidity (7). All of the reported benefits must be weighed in light of the fact that cost associated with RATS has consistently been shown to exceed that of VATS and thoracotomy. Nonetheless, the proponents of RATS often counter that the reduced length of stay offsets the higher procedural costs (4,8-11). Since the majority of lung resections being performed in the US are for presumed or suspected lung cancer, it is important to also note that the oncologic outcomes associated with RATS have demonstrated non-inferiority compared to either thoracotomy or VATS lobectomy (8,12-14).
Barriers to adoption
High initial capital investment, ongoing costs, and length of operative times are common perceived disincentives to adoption of robotic programs. Successful performance of a new technical task requires two elements: mastery of domain knowledge and mastery of technical knowledge. An experienced open surgeon possesses the domain knowledge, but to achieve competency in technical knowledge the surgeon needs to practice either in a simulated environment or under mentorship in a clinical environment, much akin to training of a novice surgeon. However, a resident/fellow learning a robotic procedure will need to acquire both domain and technical knowledge. The remaining text will highlight the transition to robotic surgery for the experienced surgeon and briefly describe special considerations for resident trainees or the novice surgeon.
The learning curve for an experienced surgeon in transitioning to robotic surgery ranges from 18–22 cases by most reports (15-21). These estimates are primarily based on proxies for competency including surgeon operative time, blood loss, and conversion to open thoracotomy. Most of these metrics do not consider the experience of the surgeon nor the changes in surgeon comfort level over time which can have a major impact on surgical success. Some have attempted to examine the effect of surgeon background on transition metrics by categorizing surgeons as either experienced VATS versus open thoracotomy surgeons. While most reports in the literature focus on converting a practice from VATS to RATS (rather than purely open thoracotomy background), there are studies that suggest that transition to robotics may be more efficient coming from a predominantly open thoracotomy background than a surgeon who attempts to convert to VATS first. Feczko found that initial proficiency defined as an operative time target of 250 minutes was 40% for novice surgeons, 14% for experienced open-to-robotic surgeons and 21% for experienced VATS-to-robotic surgeons. After the learning curve of 20 cases, however, most were proficient regardless of background of experience (novice, 93%; open-to-robotic, 100%; and VATS-to-robotic, 86%) (22).
Having weighed the pros and cons of adopting robotic surgery into their thoracic surgery practice, the surgeon should only embark on a robotic training course when they are fully committed to incorporating robotic surgery into their practice as routine. As with the performance of any technical task, repetition is key as evidenced by the learning curve described above. That being said, surgeons must be prepared to undergo a full robotic training curriculum including dry labs, cadaver labs, and case observations that must be completed prior to performing the first case. Additionally, the training itself can be a costly undertaking when one considers the cost of course fees, travel, and time away from clinical cases at home. Many surgeons who begin training and fail to achieve a comfortable level of autonomy to successfully incorporate robotics into their practice do so due to time commitment constraints.
Another potential barrier to successful transition to robotic thoracic surgery is concern for patient safety. The same features of the robotic platform which contribute to the reported benefits namely, computer-assisted dissection, actually place the surgeon one step further removed from the patient. Perceived lack of control and inability to immediately respond to an intra-operative misadventure are enough to hamper the enthusiasm of even the most experienced surgeon. Thankfully, reports in the literature fail to consistently validate these concerns with low rates of conversion to thoracotomy for uncontrolled bleeding ranging from 1–19% (5). The most common reasons for conversion are incomplete interlobular fissure, adhesions, and a prolonged operative time (23). More reassuringly is that the incidence of the above do not seem to be significantly higher during the learning curve, suggesting that robotic lobectomy can be done safely even during the transition period (24).
Robotic training
Options for the practicing surgeon
There are several options for training in robotic surgery. Academic fellowships tend to take the form of a 1-year commitment while mini-fellowships span 1–2 weeks, and mentored skill courses consist of varying lengths which offer a more tailored approach. Which program to embark upon will depend on the surgeon background, immediacy of potential cases, financial considerations and time commitment. There have been various efforts by institutions and professional surgical societies towards the creation and implementation of a standard robotic curriculum. None of the programs have had the benefit of widespread adoption despite their reported success.
Intuitive Surgical provides a comprehensive set of training courses for surgeons and other medical professionals using the da Vinci Surgical System. These in-depth educational offerings include programs focused on core technology as well as a progressive surgeon-led series focused on clinical skills advancement. The courses start with graduated modules beginning first with didactics, followed by dry lab work using simulators and familiarity with the system components, instrumentation, docking, and undocking and simple dexterity (Table 1). Once these pre-requisites are met, wet labs are performed based on a standard operation (pelvic surgery) followed by specialty specific cadaver labs. Additional advanced courses are offered to refine technique and supplemented by case observations. Case observations can be done in-person or remotely if one is near a center that offers this option.

Full table
Recognizing the existence of other competitive robotic platforms, the Fundamentals of Robotic Surgery (FRS) was created as a basic robotic surgery skills training course for surgeons that was “robotic-system agnostic” (e.g., not specific to one system), independent of industry influence, and limited to systems requiring total control by the surgeon. In addition, the FRS was designed to cover skills required by the surgeon and surgical team from the moment the patient enters the operating room until they exit the operating room. It is an “open source” course (www.frsurgery.org) to be adopted, adapted, or reconfigured to suit requirements of those in need of a resource for robotic surgery training and assessment of basic robotic skills. It has been validated in one randomized control trial demonstrating non-inferiority to other traditional robotic training methods (25).
Training of the surgical team & dealing with disaster
Any successful surgeon understands that surgery is a team sport. Developing a robotic thoracic surgery program is no different from developing any other surgical specialty or new procedure within a specialty. Once the resources have been secured from the hospital or institution, it is imperative that a dedicated team be identified and that those members are allowed the time required to complete team training and ensure safe deployment of the program. At minimum, multidisciplinary team members should include anesthesiologists, nurses, surgical technicians, physician assistants, surgical first assists and in the case of cardiac surgery: perfusionists with knowledge of minimally invasive cannulation (26). It is not recommended to have alternating team-members in the starting phase, as it will slow down the entire process with potential deleterious effects for the team and for the patient. Ideally, team members will have experience in thoracic surgery such that the main subject matter is a known-entity although there is potential benefit from drawing from staff with cross-over experience from other specialties with robotic surgery already in use.
The nursing staff are of particular importance, as they are needed to configure the appropriate instrumentation and troubleshoot any technical difficulties with the equipment during the procedure. A well-trained nursing staff allows the console surgeon to focus completely on the technical aspects of the procedure. Robotic surgery is unique in that the primary surgeon is not at the operating field. As a result, the training and knowledge of the first assistant and the nursing staff play a larger role in these procedures. Bedside assistants and nursing staff must be prepared for cases requiring emergency thoracotomy with training in emergency patient cart/robot detachment to ensure that they are able to detach the robot within 15 seconds when necessary. All team members should be engaged in the development of an emergency protocol and time should be dedicated to practice for the infrequent occurrence of intra-operative complications. The da Vinci system also requires that the facility have one nurse or technician who has taken the certification course approved by Intuitive Surgical, Inc. as part of the staff. With this in mind, the RATS perioperative nursing development program was created which includes environment, patient safety, and surgical team management as the three primary objectives. These three categories incorporate the required knowledge and skills that enable effective management of a safe surgical experience for the patient undergoing RATS (27).
Technical considerations
Case and patient selection
Patient selection is key when embarking on a new surgical program in order to maximize patient safety. It may be a costly endeavor to perform “simple cases” such as wedge resections, small pleural mass resections and bullectomy using a robotic system, yet doing so shortens the learning curve and enhances technical skills acquisition by prioritizing technical competence with the instruments over that of performance of the case itself. Additionally, when performing the initial lobectomies, one should consider those patient characteristics that elevate surgical risk. For instance, age, gender, and stage have not been associated with increased rates of conversion to thoracotomy, but BMI has been noted as a significant risk factor for conversion (28). Patients with hilar lymphadenopathy are also poor candidates for initial robotic lobectomy cases. Focusing on smaller cases initially will increase the volume of cases available and thus maximize the repetition and deliberate practice required for competence. Having a list of cases with graduated complexity will also help during the transition. A useful guide to case complexity categories for the surgeon in transition can be found in Table 2. Thus, limiting initial cases to small resections on low-risk patients will ensure quick “wins” for the team in measuring success of the robotic program.
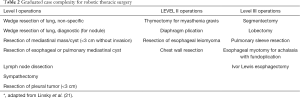
Full table
Case component/segmentation
When performing the first robotic lobectomy, it is important see the benefit of case segmentation as an aid to training. By breaking down a given case into smaller components, the surgeon can concentrate on 1–2 main steps (e.g., lymphadenectomy or division of the pulmonary vein) that can be mastered before moving on to tackle all steps of the operation. This strategy is also helpful for time management as surgeons may find themselves absorbed in a single step with slow progress losing sight of the time that has elapsed leading to lengthy surgical and anesthetic times for the patient. Added to this is the potential for worsening surgeon performance as a case goes longer in duration. When using case segmentation as a training tool, it is important to determine the steps that will be done robotically prior to the operation. The latter is also communicated to the operating room team who then understands what is going to be done, so that they can be prepared and not lose confidence during the transition.
Other assistance
Product and technical representatives from robotic companies can be a valuable resource to the surgeon in transition. They have in-depth technical knowledge and help to train both the surgeon and other members of the team. They can provide important technical advice for trouble-shooting common problems encountered during the course of a case which greatly enhances the efficiency of the team and conduct of the operation. Ideally, they are knowledgeable about the surgeon’s training pathway in terms of what has been done and where there are any remaining deficits offering resources to help address the latter.
Both novice and experienced open surgeons require supervision and mentoring during the initial phases of robotic surgery skill acquisition. The experienced open surgeon possesses domain knowledge, however, they need to acquire technical knowledge under supervision (either in simulated or clinical environment) to successfully transition to robotic surgery, whereas, novice surgeons need to acquire both domain knowledge as well as technical knowledge to become competent. Proctoring is required for both types of surgeons before one can be considered truly competent. The proctor may be facilitated by the robotic manufacturer or instead may be dictated by the institution. In the case of the latter, a solo novice thoracic surgeon may need to draw upon knowledgeable surgeons in other surgical fields at the institution (e.g., experienced Gynecology and Urologic robotic surgeons).
Surgical details
There are many available resources which outline the technical aspects of performing a lobectomy robotically including nuanced differences for each lobe. Aside from the basics of port placement, robot docking, and isolation of anatomic structures, much of the described differences are relatively subtle. Each robotic platform will require special considerations for port placement based on camera and angles of dissection. Other important differences are based upon availability of instruments (resources and finances) and surgeon familiarity with various approaches.
For those surgeons coming from a predominantly open thoracotomy background with little routine use of VATS for lobectomy, the surgical field of view and overall approach may actually seem more familiar than for those predominant VATS surgeons. Traditionally, open lobectomies are approached either via the fissure in the case of a lower lobectomy or from a posterior approach for an upper lobectomy. Either surgical view lends itself well to RATS approaches to lobectomy. By contrast, many VATS lobectomies are performed from an anterior, fissure-less technique which can be replicated with the robotic approach but may be less appealing for the open thoracotomy surgeon.
Other technical differences to note include the mode of dissection and instrumentation. Most surgeons have a preferred dissection technique whether it is primarily blunt or sharp and in the case of the latter, consideration of preferred energy source is also a factor. All options are available to the robotic surgeon. Bipolar cautery is probably the most common energy source for primary dissection in most RATS lobectomies. The majority of thoracic surgeons, however, do not routinely use bipolar cautery thus its optimal use must be mastered as a separate task. Similarly, some surgeons (including the author) favor blunt dissection using a suction tip, or peanut and right-angled clamp when performing VATS lobectomy. During the course of a robotic lobectomy, suction has traditionally been controlled by the bedside assistant thus removing this as an effective means of dissection for the console surgeon. Most recently, however robotic suction instruments have been introduced which enable direct surgeon control both for primary suction of fluid as well as use of the suction for bunt dissection. Similarly, the transitioning surgeon must take on what may be a “novel” role as camera-operator as they transition from VATS to RATS. In doing so, they must actively modify and optimize the view of the operative field throughout the procedure as an added task. Surgeons transitioning from open thoracotomy approach may find this new role challenging, but all surgeons will develop this skill quickly and come to find they are the ideal camera-operator in that the camera movement now becomes intuitive and fluid with the conduct of the operation.
Finally, the importance of the bedside surgical assistant cannot be overemphasized. As robotic platforms place the surgeon one order removed from the patient the bedside assistant role is elevated in that they are the first “hands” in the operative field in the setting of misadventure. Furthermore, they often perform tasks traditionally assigned to the primary surgeon (e.g., stapling, independent retraction, specimen retrieval). As such, a surgeon in transition may benefit from requesting a senior robotic surgeon or non-robotic thoracic surgeon to serve in this role during initial cases until an appropriate level of confidence is achieved. The “reassignment” of surgeon and assistant duties may be uncomfortable to some at first, but with time will enhance the team dynamics.
Special considerations
Institutional considerations
Before starting a robotic program, it is imperative to have institutional support to provide the appropriate infrastructure including capital investment, time for team training, and stable access to the system. A full discussion of institutional cost, capital investment, market share and contracting will differ based on existing infrastructure and are beyond the scope of this report. The question of surgeon access to robotic equipment is critical. This can be one of the most common reasons for failure to successfully implement a robotic program in a specialty if there is insufficient access to the system in order to achieve the requisite deliberate practice and generate enough return on investment. Given the widespread adoption of robotic surgery within the US, many hospitals will have pre-existing robotic platforms and infrastructure which will need to be refined to accommodate a robotic thoracic surgery program. While this bodes well in terms of resources, it may, in fact introduce competition if resources are limited. Most institutions with robotic surgery have a robotic committee or Operating Room committee charged with allocating block time and scheduling. A thoracic surgeon transitioning into robotics must familiarize themselves with the appropriate personnel that administer to the robotic program or create one de novo if none is in place. In doing so, they maintain a seat at the negotiating table to ensure proper consideration of thoracic surgery among the various potential competing interests.
Credentialing
Currently, two pathways for robotic credentialing exist for residency and non-residency-trained surgeons. In the United Sates, granting of hospital privileges falls largely to the individual institution. Each is required by the Joint Commission: Accreditation, Health Care, Certification to credential and privilege physicians on their medical staff according to local policies. Common requirements stipulate that surgeons trained in robotics during residency must fulfill 20 cases with program directors' attestation to obtain full privileges. Non-residency-trained surgeons are required to fulfill simulation, didactics including online modules, wet laboratories (cadaver or animal), and observation of at least two cases before provisional privileges can be granted as outlined above. Often, a minimum number of cases (e.g., 10 per year) are required to maintain privileges. All procedures are monitored via departmental Quality Assurance/Quality Improvement Committee review (29). Currently, the FDA recommends that physicians, hospitals and facilities that use robotic assisted surgery devices should ensure that proper training is completed and that surgeons have appropriate credentials to perform surgical procedures with these devices. Device users should ensure they maintain their credentialing. Hospitals and facilities should also ensure that other surgical staff that use these devices complete proper training (30).
Teaching trainees
Much of the training courses (including those sponsored by robotic system manufacturers) are geared towards the experienced surgeon seeking to add robotic surgery to their existing armamentarium. Recently, however more attention is being paid to creating standard curricula for the surgical trainee. One example of standardized training for residents was developed in Europe in 2016. A working panel was created among members of the European Society of Thoracic Surgeons (ESTS) and European Association for Cardio-Thoracic Surgery (EACTS) focused on training in robotic thoracic surgery. They used Delphi methodology to create a consensus opinion for standardizing robotic training to be divided into clearly defined sections as a staged learning pathway. The basic training includes a baseline evaluation, an e-learning module and simulation training. Advanced training is recommended to include video demonstrations, simulation training, hands-on modular console training, proctoring and a final evaluation of submitted video reviewed by independent examiners (31).
This standard resident training has not been universally adopted here in the US. At minimum, however, the resident trainee should undergo dedicated training including dry lab and simulation training. Several studies have shown the benefits of simulation and deliberate practice which should be compulsory before the trainee is allowed to progress towards scrubbing as the bedside assistant and certainly before moving on to be the console surgeon (32,33). Once the resident is deemed ready to serve as the console surgeon, the presence of a dual console is invaluable in making that transition. The dual console was first introduced into the da Vinci system in 2009 and has become a core element for safely granting graduated responsibility for the surgical trainee. Its use among thoracic surgery training programs is not universal, however with some prevalence estimates of 70% within integrated 6-year training programs (34). The dual console allows the trainee to act as the console surgeon alongside an experienced console surgeon sitting next to them for guidance throughout the operation. The trainee can see directly, instantaneously, and in the same orientation how that step should be performed. Sections of the operation can be done as described above for case segmentation and subsequently the trainee can proceed towards performing an entire operation. Moreover, taking controls from the trainee by the attending surgeon does not involve switching positions, which lengthens the operation, or performing the maneuver from the opposite side. Thus, the dual console is another high value tool for the experienced non-robotic surgeon making the transition, the novice trainee, as well as the experienced robotic surgeon seeking to improve their operative teaching techniques.
Conclusions
The introduction of robotic surgery into a thoracic surgery practice requires significant investment in terms of time and resources from the surgeon as well as the institution. Skill development must proceed in a defined manner with deliberate practice including simulation training and graduated levels of case complexity. The educational pathway may differ slightly depending on the background (VATS or open thoracotomy) and experience (novice or seasoned) of the surgeon, but the main elements above remain the same. With all of the above elements in place, one can safely acquire and master robotic thoracic surgical techniques in a relatively short period of time.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Natalie S. Lui and Sean C. Wightman) for the series “Robotic Surgery for Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2020.01.09). The series “Robotic Surgery for Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- iData Research. Robotic Surgery Statistics Show Movement Towards More Minimally Invasive Procedures. 2018. Available online: https://idataresearch.com/robotic-surgery-statistics-show-movement-towards-more-minimally-invasive-procedures/. Accessed 12/23/2019.
- Childers CP, Maggard-Gibbons M. Estimation of the Acquisition and Operating Costs for Robotic Surgery. JAMA 2018;320:835-6. [Crossref] [PubMed]
- Zirafa CC, Romano G, Key TH, et al. The evolution of robotic thoracic surgery. Ann Cardiothorac Surg 2019;8:210-7. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Mahieu J, Rinieri P, Bubenheim M, et al. Robot-Assisted Thoracoscopic Surgery versus Video-Assisted Thoracoscopic Surgery for Lung Lobectomy: Can a Robotic Approach Improve Short-Term Outcomes and Operative Safety? Thorac Cardiovasc Surg 2016;64:354-62. [PubMed]
- Nasir BS, Bryant AS, Minnich DJ, et al. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg 2014;98:203-8; discussion 208-9. [Crossref] [PubMed]
- Guo F, Ma D, Li S. Compare the prognosis of Da Vinci robot-assisted thoracic surgery (RATS) with video-assisted thoracic surgery (VATS) for non-small cell lung cancer: A Meta-analysis. Medicine (Baltimore) 2019;98:e17089 [Crossref] [PubMed]
- Nelson DB, Mehran RJ, Mitchell KG, et al. Robotic-Assisted Lobectomy for Non-Small Cell Lung Cancer: A Comprehensive Institutional Experience. Ann Thorac Surg 2019;108:370-6. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 42-4. [Crossref] [PubMed]
- Worrell SG, Dedhia P, Gilbert C, et al. The cost and quality of life outcomes in developing a robotic lobectomy program. J Robot Surg 2019;13:239-43. [Crossref] [PubMed]
- Kneuertz PJ, Singer E, D'Souza DM, et al. Hospital cost and clinical effectiveness of robotic-assisted versus video-assisted thoracoscopic and open lobectomy: A propensity score-weighted comparison. J Thorac Cardiovasc Surg 2019;157:2018-26.e2. [Crossref] [PubMed]
- Toosi K, Velez-Cubian FO, Glover J, et al. Upstaging and survival after robotic-assisted thoracoscopic lobectomy for non-small cell lung cancer. Surgery 2016;160:1211-8. [Crossref] [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [Crossref] [PubMed]
- Cerfolio RJ, Ghanim AF, Dylewski M, et al. The long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. [Crossref] [PubMed]
- Veronesi G. Robotic thoracic surgery: technical considerations and learning curve for pulmonary resection. Thorac Surg Clin 2014;24:135-41. v. [Crossref] [PubMed]
- Cheufou DH, Mardanzai K, Ploenes T, et al. Effectiveness of Robotic Lobectomy-Outcome and Learning Curve in a High Volume Center. Thorac Cardiovasc Surg 2019;67:573-7. [Crossref] [PubMed]
- Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 2008;18:289-95. vi-vii. [Crossref] [PubMed]
- Lee BE, Korst RJ, Kletsman E, et al. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg 2014;147:724-9. [Crossref] [PubMed]
- Meyer M, Gharagozloo F, Tempesta B, et al. The learning curve of robotic lobectomy. Int J Med Robot 2012;8:448-52. [Crossref] [PubMed]
- Linsky PL, Wei B. Training in robotic thoracic surgery. J Vis Surg 2018;4:1. [Crossref] [PubMed]
- Feczko AF, Wang H, Nishimura K, et al. Proficiency of Robotic Lobectomy Based on Prior Surgical Technique in The Society of Thoracic Surgeons General Thoracic Database. Ann Thorac Surg 2019;108:1013-20. [Crossref] [PubMed]
- Nakamura H. Systematic review of published studies on safety and efficacy of thoracoscopic and robot-assisted lobectomy for lung cancer. Ann Thorac Cardiovasc Surg 2014;20:93-8. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Starting a robotic program in general thoracic surgery: why, how, and lessons learned. Ann Thorac Surg 2011;91:1729-36; discussion 36-7.
- Satava RM, Stefanidis D, Levy JS, et al. Proving the Effectiveness of the Fundamentals of Robotic Surgery (FRS) Skills Curriculum: A Single-blinded, Multispecialty, Multi-institutional Randomized Control Trial. Ann Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Rodriguez E, Nifong LW, Bonatti J, et al. Pathway for surgeons and programs to establish and maintain a successful robot-assisted adult cardiac surgery program. J Thorac Cardiovasc Surg 2016;152:9-13. [Crossref] [PubMed]
- Sarmanian JD. Robot-Assisted Thoracic Surgery (RATS): Perioperative Nursing Professional Development Program. Aorn j 2015;102:241-53. [Crossref] [PubMed]
- Baldonado JJ, Amaral M, Garrett J, et al. Credentialing for robotic lobectomy: what is the learning curve? A retrospective analysis of 272 consecutive cases by a single surgeon. J Robot Surg 2019;13:663-9. [Crossref] [PubMed]
- Bhora FY, Al-Ayoubi AM, Rehmani SS, et al. Robotically Assisted Thoracic Surgery: Proposed Guidelines for Privileging and Credentialing. Innovations (Phila) 2016;11:386-9. [Crossref] [PubMed]
- US FDA. Computer-Assisted Surgical Systems. 2019. https://www.fda.gov/medical-devices/surgery-devices/computer-assisted-surgical-systems#3 Accessed 12/23/19
- Veronesi G, Dorn P, Dunning J, et al. Outcomes from the Delphi process of the Thoracic Robotic Curriculum Development Committee. Eur J Cardiothorac Surg 2018;53:1173-9. [Crossref] [PubMed]
- Culligan P, Gurshumov E, Lewis C, et al. Predictive validity of a training protocol using a robotic surgery simulator. Female Pelvic Med Reconstr Surg 2014;20:48-51. [Crossref] [PubMed]
- Schreuder HW, Persson JE, Wolswijk RG, et al. Validation of a novel virtual reality simulator for robotic surgery. ScientificWorldJournal 2014;2014:507076 [Crossref] [PubMed]
- Raad WN, Ayub A, Huang CY, et al. Robotic Thoracic Surgery Training for Residency Programs: A Position Paper for an Educational Curriculum. Innovations (Phila) 2018;13:417-22. [Crossref] [PubMed]
Cite this article as: Backhus LM. Transitioning from VATS to robotic lobectomy. Video-assist Thorac Surg 2020;5:19.


