Advanced robotic resections in lung cancer
Advantages of minimally invasive approach
Treatment of locally advanced lung cancer presents multiple layers of complexity to the thoracic surgeon. These patients frequently require a multi-modal approach, which may require a wide array of treatment including chemotherapy, radiation therapy, or immunologic therapy in the neo-adjuvant or adjuvant setting. All these therapies along with bulky or invasive tumors make operative planning challenging. Many surgeons tread carefully and revert to open technique approaches in an effort to maximize exposure and deal with the unexpected. Minimally invasive techniques for lung cancer resections have become more widely adopted and sophisticated over time. The shift towards a minimally invasive approach has been fueled by reduction in mortality, hospital stay, and overall complication rates (1). Robotic lung surgery has been further associated with shorter hospital stays, improved 30-day mortality, and less post-operative transfusions relative to video-assisted thoracoscopic surgery (VATS) and thoracotomy (2). Conferring these benefits of a robotic assisted approach to patients with locally invasive disease is both safe and manageable in properly selected patients (3).
Benefits of robotics
From a technical standpoint, the robotic platform provides the surgeon with multiple tools that embolden his or her ability to complete the resection safely and more effectively. This platform improves visualization of the surgeon by providing real stereovision in high definition. The image is high definition, 3-dimensional, and magnified 10-fold, which exposes many of the subtleties of the tissue to the surgeon’s eye. Use of a 30-degree lens also gives view to aspects of the anatomy that cannot be visualized from an open approach. This advantage over an open incision is most apparent when trying to visualize behind or deep to fixed structures, such as the tumor or airway. These areas are difficult to see through a thoracotomy; therefore, visualization is improved. Additionally, the robotic platform’s wristed instruments enable the surgeon to cautiously dissect anatomical structures especially in small confined areas within the chest cavity. Neoadjuvant therapy, especially immunotherapy, is associated with an intense inflammatory response within the lymph nodes. This makes dissection between nodes and the vascular structures of the lung more treacherous. The robotic platform can also be used to enhance a surgeon’s dexterity by utilizing software to translate movements into a finer form. With the improved visualization and small dexterous instrumentation, the robotic approach enhances the surgeon’s ability to safely dissect lymph nodes and pulmonary structures in a smaller confined space. Some retrospective reviews have associated robotic lung resections with safer and more effective outcomes when compare to VATS lung resection (4).
Our approach
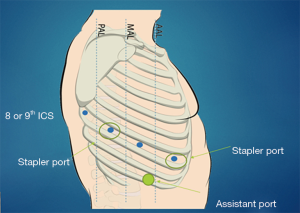
Patients at our institution are discussed at a multidisciplinary collaborative meeting to develop an optimal individualized treatment plan. In the following sections, we will discuss specific approaches for patients undergoing neoadjuvant therapy and technical considerations for patients undergoing sleeve resections, chest wall resections, or pneumonectomy. We use the Da Vinci Xi platform from Intuitive Surgical (Sunnyvale, CA, USA) for our lung resections. Our general docking approach for patients with locally advanced tumors does not deviate tremendously from our standard approach. After conducting a review of all updated pre-operative imaging, we practice careful entry into the chest. Camera port is placed anterior to the tip of the scapula at the eighth or ninth intercostal space with an 8-millimeter port. The remaining ports are placed under direct vision. As the anatomy can be distorted by adhesions and mass effect, predicting the angles for safe stapling can be difficult so we place one or two 12-millimeter staple ports, one anterior in the chest and the other approximately eight centimeters posterior to the initial port to allow bidirectional stapling. The fourth arm is docked approximately 8-centimeters posterior to the stapler port, but with care not to be too close to the spine (usually 4 centimeters lateral to the spine). Lastly, a 15-millimeter assistant port is triangulated two rib spaces below the two anterior ports usually at the tenth intercostal space. Use of a 15-millimeter port for the bedside assistant which is also the site of specimen extraction. This universal approach can be seen in Figure 1 above. Depending on which lobe is being resected, use of only one stapling port may be sufficient. Generally, resection of lower lobes only necessitates the anterior stapler port and resection of the middle may only necessitate the posterior stapler port. Upper lobe resections are more likely to require bidirectional stapling and necessitate both stapler ports.
Lung resection after chemo/XRT/immunotherapy
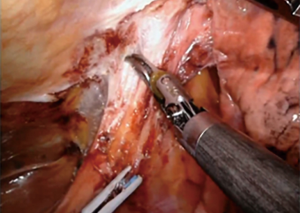
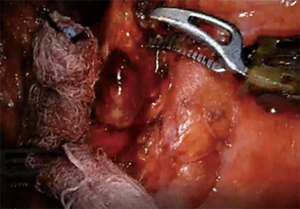
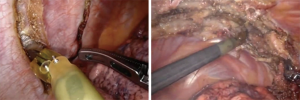
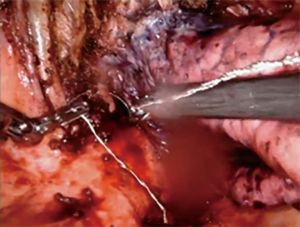
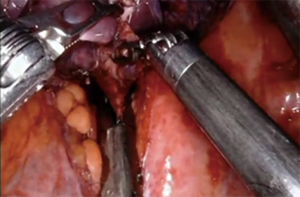
For patients having undergone neoadjuvant therapy, initial entry into the chest can be complicated by significant adhesions (Figure 2). Space creation with a harmonic scalpel or similar energy device may be necessary to allow for placement of ports in the appropriate space. Once docked, it is key to use bipolar energy to fully mobilize the lung from adhesions. We have found good success with a posterior approached dissection of the hilum, which means as the individual structures of the hilum are freed and dissected posteriorly, we will then proceed with stapling and transection in a stepwise fashion. This affords more mobility and better visualization in an otherwise challenging field. Use of bidirectional stapling is important to achieve this step. With immunotherapy, lymph node dissection can be exceedingly more difficult due to inflammation of nodes and adherence to surrounding vasculature (Figure 3). Meticulous dissection along with transection of deep nodes adherent to the pulmonary artery is often needed to complete the dissection safely. Visualization is generally improved with the robotic platform and the difficulty is not abated in the open setting, which is why careful, patient progression is often the best option.
Stereotactic body radiation therapy (SBRT) frequently causes an intense induration and scaring of the lung. This finding is more pronounced than with conventional radiation. Resection after failed SBRT can still be approached robotically, but may require a larger resection to remove all the damaged lung. Anytime we suspect the bronchus has been radiated, it is our practice to cover the stump with an intercostal muscle flap.
Techniques for sleeve
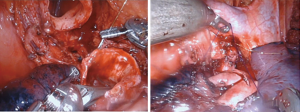
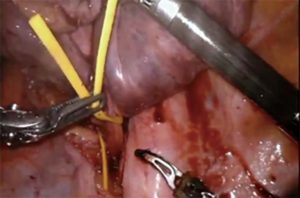
The sleeve bronchoplasty procedure offers patients an alternative to a pneumonectomy with a significantly better morbidity and mortality profile (5-7). The key to this procedure is the reconstruction. In the minimally invasive setting, some surgeons have found the robotic platform to have an easier learning curve to perform the anastomosis (8). Robotic sleeve resections have been demonstrated to be safe in properly selected patients (9). Our technique for sleeve resections is similar to that of a standard lobectomy. Once the lobe in question is completely mobilized and the vascular structures divided, we use the robotic shears to divide the bronchus to grossly negative margins. The proximal bronchial division is made as close as possible to the origin of the main bronchus. Distal bronchial division is close to the lobar segmental subdivision. The distal lobe must be adequately mobilized for anastomosis. While a complete nodal resection is important with clearance of mediastinal nodes, it is also extremely important not to devascularize the bronchi involved in the resection. The robot allows for simplified intracorporeal suturing (Figure 4). We typically use an absorbable suture to run the membranous portion and interrupted absorbable suture for the cartilaginous segment. For efficiency, we have had success using a barbed monofilament suture, which requires less knot tying and holds a consistent tension when bring the anastomosis together. Use of a monopolar spatula can help facilitate harvest of a well vascularized intercostal bundle from an intercostal level corresponding to the anastomosis. Harvest is improved by skeletonizing the ribs above and below the bundle (Figure 5). The pedicle is harvested from anterior to posterior until the base is near the spine to allow for adequate length. The pedicle is then placed between the anastomosis and pulmonary artery and sutured in place to avoid it from slipping away after lung re-expansion. Alternatively, the pericardial fat pad can be utilized as a tissue flap between the two structures.
Techniques for chest wall resections
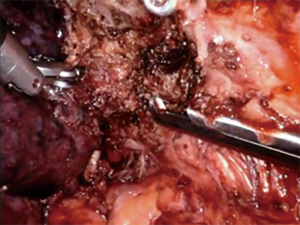
Tumors invading the chest wall require extra inspection of the pre-operative imaging. Docking the robot as low as possible can improve the angulation of the instruments for successful dissection. As the robot is docked low in the chest, use of a robotic approach is not advisable for tumors with chest wall invasion below the seventh rib. As discussed above, a posterior dissection of the hilum can be helpful for apical and anterior masses. Anterior stepwise dissection may be needed for low posterior masses. Once the hilum is completely free, we use energy to define the dissection margin by obtaining circumferential control of the rib level above and below the mass (Figure 6). With the resection and ribs demarcated, there are several approaches to resect the mass. First, and perhaps the easiest, an incision can be made over the involved area and the ribs can be divided with open instruments. If the mass is not easily accessible externally, a gigly saw can be used in coordination with two robotic needle drivers to transect the medial and lateral ends of the involved ribs for resection (Figure 7). Internal dissection and resection allow for preservation of the chest wall musculature and integrity. This approach is limited in the anterior chest due to limited mobility from the instruments. Lastly, for ribs that are in line with a port to be resected with straight thoracoscopic instruments, Dennis rib cutters or Kerrison rongeurs can be used to divide the ribs (Figure 8). Frequently, the extraction site must be enlarged to accommodate the ribs extracted. It is best to extract where there is least compromise of the chest wall.
Pneumonectomy
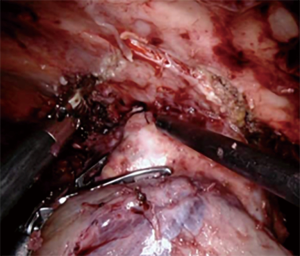
Large central hilar masses on occasion necessitate pneumonectomy. While the full benefits of minimally invasive resection for pneumonectomy have not been clearly elucidated in the literature, evidence does support that the minimally invasive approach is safe and confers a similar oncologic outcome as thoracotomy (10). Pneumonectomies have become rare but are often needed for large central masses and masses that cross the fissure in the left lung. Our experience has been principally with left pneumonectomies as the three lobes of the right lung allow for more resection options. A tumor encircling the main pulmonary artery is the main contraindication to a robotic approach as proximal control and stapling within the mediastinum is difficult to accomplish. It is our experience to elongate the hilum and perform a complete lymph node dissection to give better access to the base of the hilar vessels. Posterior dissection is completed first with lymph node dissection and dissection of the posterior aspect of the pulmonary artery trunk. This will facilitate easier dissection of the pulmonary artery when dissecting from the anterior hilum later. Then we dissect and circumferentially control the inferior pulmonary vein and use a vessel loop as a place holder to facilitate rapid encircling at time of division (Figure 9). Next the pericardium is incised lateral to the phrenic and the superior pulmonary vein is encircled with a vessel loop. Anterior dissection of the pulmonary artery is started to ensure the division can be completed safely and with a grossly negative tumor margin. The base of the pulmonary artery cannot be typically cleared until the superior vein is divided. We then divided the inferior and superior pulmonary veins to allow for complete exposure of the artery base, which is then circumferentially dissected to meet our posterior dissection. The artery is then divided. With the pulmonary artery and veins divided, traction is placed on the mainstem bronchus with a grasper during stapling to avoid a long bronchial stump (Figure 10). An intercostal muscle flap is placed over the bronchial stump. Specimen is extracted with a large reinforced thoracoscopic bag through the assistant port. Skin incision is widened appropriately to accommodate the specimen.
Considerations for non-robotic approaches
We always try to offer our patients a minimally invasive resection when possible. Use of the robot has improved our ability to offer this. We feel more comfortable proceeding with an open approach with specific patients. Tumors invading hilar vascular structures present a challenge to a robotic approach as proximal vascular control can be difficult. Use of an additional port medially with a Rommel tourniquet or vessel loop under direct control of bedside assistant is a workable strategy. When dissecting on a hilar tumor with difficult proximal vascular control, it is often better to convert to an open approach. If a tumor encompasses more than half the circumference of the main pulmonary artery, it has been our practice to proceed directly to a thoracotomy for resection with vascular sleeve.
Conclusions
The use of a robotic platform offers improved visualization and dexterous instrumentation for a surgeon completing complex lung resections. These approaches have aided us in providing our patients with locally advanced disease with the benefits of a minimally invasive approach. These patients frequently require additional systemic therapy or radiotherapy, which is often impeded by the added morbidity from open lung resections. Continually improving our skills and using helpful tools has an immediate impact on the patient experience and will ultimately manifest in additional years of quality life for our patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Natalie S. Lui and Sean C. Wightman) for the series “Robotic Surgery for Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2020.01.04). The series “Robotic Surgery for Lung Cancer” was commissioned by the editorial offce without any funding or sponsorship. Dr. Schumacher is a proctor and speaker for Intuitive Surgical (Sunnyvale, CA, USA). The authors have no conficts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Farivar AS, Cerfolio RJ, Vallières E, et al. Comparing robotic lung resection with thoracotomy and video-assisted thoracoscopic surgery cases entered into the Society of Thoracic Surgeons database. Innovations (Phila) 2014;9:10-5. [Crossref] [PubMed]
- Veronesi G, Park B, Cerfolio R, et al. Robotic resection of Stage III lung cancer: an international retrospective study. Eur J Cardiothorac Surg 2018;54:912-9. [Crossref] [PubMed]
- Li JT, Liu PY, Huang J, et al. Perioperative outcomes of radical lobectomies using robotic-assisted thoracoscopic technique vs. video-assisted thoracoscopic technique: retrospective study of 1,075 consecutive p-stage I non-small cell lung cancer cases. J Thorac Dis 2019;11:882-91. [Crossref] [PubMed]
- Abdelsattar ZM, Shen KR, Yendamuri S, et al. Outcomes After Sleeve Lung Resections Versus Pneumonectomy in the United States. Ann Thorac Surg 2017;104:1656-64. [Crossref] [PubMed]
- Okada M, Tsubota N, Yoshimura M, et al. Extended sleeve lobectomy for lung cancer: the avoidance of pneumonectomy. J Thorac Cardiovasc Surg 1999;118:710-3; discussion 713-4. [Crossref] [PubMed]
- Cusumano G, Marra A, Lococo F, et al. Is sleeve lobectomy comparable in terms of short- and long-term results with pneumonectomy after induction therapy? A multicenter analysis. Ann Thorac Surg 2014;98:975-83. [Crossref] [PubMed]
- Pan X, Gu C, Wang R, et al. Initial Experience of Robotic Sleeve Resection for Lung Cancer Patients. Ann Thorac Surg 2016;102:1892-7. [Crossref] [PubMed]
- Jiao W, Zhao Y, Qiu T, et al. Robotic Bronchial Sleeve Lobectomy for Central Lung Tumors: Technique and Outcome. Ann Thorac Surg 2019;108:211-8. [Crossref] [PubMed]
- Yang CJ, Yendamuri S, Mayne NR, et al. The role of thoracoscopic pneumonectomy in the management of non-small cell lung cancer: A multicenter study. J Thorac Cardiovasc Surg 2019;158:252-264.e2. [Crossref] [PubMed]
Cite this article as: Hasan OF, Schumacher L. Advanced robotic resections in lung cancer. Video-assist Thorac Surg 2020;5:9.