Long-term outcomes of 3-port VATS lobectomy with selective mediastinal lymphadenectomy for clinical stage I non-small cell lung cancer
Introduction
In the last few years, video-assisted thoracic surgery (VATS) has been gaining interest and consensus approval among thoracic surgeons worldwide, becoming the procedure of choice in an increasing number of institutions. From the annual report of the Japanese Association for Thoracic Surgery, 67% of primary lung cancers (42,282 cases) were resected thoracoscopically in 2016 (1).
Initial studies have noted that VATS lobectomy results in improved rates of peri- and postoperative complications, reductions in hospital stay, chest tube duration, and postoperative pain, and higher quality of life. These findings have mainly come from non-randomized observational studies, national databases, institutional analyses (2-4) and meta-analyses (5,6). However, results were reported in 2016 from a well-designed, randomized controlled trial (RCT) in Denmark comparing the invasiveness of VATS and anterolateral thoracotomy for early-stage non-small cell lung cancer (NSCLC) (7). In that report, VATS was shown to be associated with lower postoperative pain and better quality of life compared to anterolateral thoracotomy for the first year after surgery.
In response to growing evidence of the oncologic equivalence and reported benefits of minimally invasive surgery, VATS lobectomy has become the technique of choice for resecting early-stage lung cancers at many institutions. However, over the last decade, concerns over the oncologic efficacy and safety have limited the acceptance and uptake of this procedure. VATS can use many approaches, depending on the preferences of the individual surgeon, including in terms of the use of a rib spreader, positioning of access incisions, and viewing of the thoracic cavity directly through minithoracotomy or via a monitor. We must therefore consider the possibility of biases in patient selection and differences in VATS procedures among institutes.
This report describes the experience of 14 surgeons with 3-port access VATS lobectomy at a large academic teaching institution using a standardized technique. The objective of this study was to evaluate the efficacy of this technique with respect to oncologic validity.
Methods
Patients
Between January 2005 and December 2013, a total of 1,133 patients were enrolled for 3-port thoracoscopic lobectomy to treat various stages of primary lung cancer at Toranomon Hospital, Japan. Among those 1,133 patients during this period, 816 patients with clinical stage I NSCLC underwent 3-port VATS lobectomy with selective lymphadenectomy and were able to be followed-up for 5 years postoperatively. Patients were included in the analyzed population if they had data available for postoperative follow‐up 5 years, no second primary cancer within 5 years of the index lung cancer, and no neoadjuvant or adjuvant radiotherapy. The medical records and pertinent radiologic images of patients were reviewed to characterize demographic information and obtain surgical and pathologic details. Clinical and pathological stages were determined according to the 7th edition of the TNM Classification for Lung and Pleural Tumors (8).
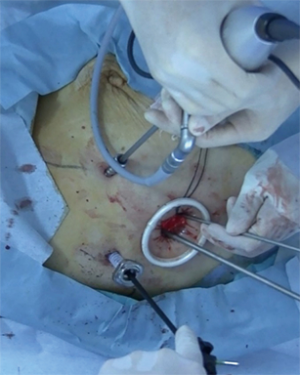
Surgical procedures for 3-port VATS
The patient was placed in the lateral decubitus position under general anesthesia with single-lung ventilation. Right-side 3-port VATS was performed via a 1.2-cm port on the mid-axillary line in the 6th intercostal space using endoscopic instruments. Intercostal muscles were minimally divided by about 2.0–3.5 cm to remove resected specimens. A 7-mm port was placed on the posterior axillary line in the 4th intercostal space for the operator’s left hand. A 5- or 10-mm port was placed on the anterior axillary line in the 4th intercostal space for the angled 30-degree thoracoscope (Figure 1). If no malignant diagnosis had been established preoperatively, thoracoscopic wedge resection or fine needle biopsy was performed initially. All procedures were strictly monitor-based and the role of each port was fixed. All major pulmonary vessels and bronchi in the affected lobe were transected using an endoscopic stapler. No metal chest retractor was used for this incision; instead, a 2.5-cm-diameter silicone rubber instrument (Alexis wound retractor XXS; Applied Medical Resources Corporation, Tokyo, Japan) was used to enable wound protection and allow more room for the assistant’s instruments for mediastinal lymphadenectomy. Exclusion criteria for 3-port VATS lobectomy for NSCLC included T3 or T4 tumors, endobronchial tumors visible at bronchoscopy, use of induction radiotherapy, and the presence of N2 disease on CT and positron emission tomography. Previous thoracic operations, incomplete fissures, and pleural adhesions were not considered contraindications for the VATS procedure. During VATS lobectomy, subcarinal lymphadenectomy is not always necessary for cancers of the right upper lobe or left upper segment. Superior mediastinal lymphadenectomy was omitted for cancers of the right lower lobe, and an aortopulmonary window and paratracheal lymphadenectomy were omitted for cancers of the left lower lobe. This technique of minimized mediastinal lymph node dissection was defined as selective node dissection. Systematic dissection of all mediastinal lymph node stations was performed for patients with cN1. Mediastinal lymph node dissection was omitted for patients 80 years old with cN0. During the study period, 14 surgeons began performing VATS lobectomy at our institute and used our training protocol applied routinely after performing pulmonary wedge resection or mediastinal tumor resection for 6 months.
Data acquisition and follow-up
In general, pathologic and surgical data were recorded for each patient. Pathologic data including pathologic type of the tumor, cell differentiation, tumor size, lymph node metastasis, pleural involvement, and microscopic pulmonary metastasis in the resected lobe were collected. A survival and disease-status census was conducted every 6 months by chest computed tomography after surgery for the first 5 years. In March 2019, survival data were updated based on information obtained from the medical records or by contacting the patient, their family, or the referring physician. Recurrence was classified as locoregional when the disease developed in ipsilateral hilar or mediastinal lymph nodes, the surgical margin, ipsilateral lung or ipsilateral pleural space (pleural dissemination or carcinomatous pleuritis). Recurrence was classified as distant when the disease emerged in distant organs, including the supraclavicular fossa or contralateral hilum. Continuous data are expressed as median (range). Survival periods were estimated using Kaplan-Meier methods from the date of surgery until detection of overall survival (OS), any recurrence [disease-free survival (DFS)], or locoregional recurrence (locoregional recurrence-free survival).
Results
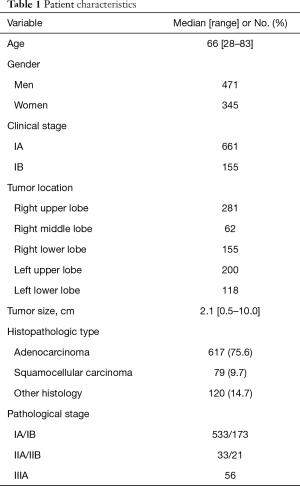
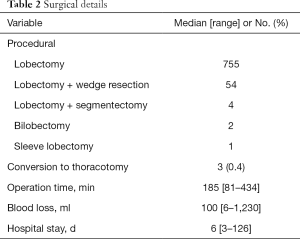
We identified 816 patients (471 men, 345 women) who underwent 3-port VATS lobectomy with selective mediastinal lymphadenectomy for clinical stage I NSCLC in the defined study period. Patient characteristics and surgical details are found in Tables 1 and 2. Three procedures (0.4%) were converted to open thoracotomy because of bleeding from a pulmonary vessel. Postoperative course was uneventful for all 3 converted cases. Median patient age was 66 years, with 152 patients 75 years old. Clinical stage was IA in 661 patients and IB in 155 patients. Primary lung cancer was diagnosed preoperatively in 18.6% of patients; bronchoscopically in 89 patients, and by CT-guided needle biopsy in 64 patients. All other patients were diagnosed intraoperatively, by needle biopsy in 265 patients, wedge resection in 240 patients, and lobectomy in 158 patients. Lung resection comprised lobectomy in 755 patients, lobectomy with wedge resection in 54, lobectomy with segmentectomy in 4, bilobectomy in 2, and sleeve lobectomy in 1. Median operation time was 185 min, and median blood loss was 100 mL. Median postoperative stay was 6 days. No re-thoracotomy was required for hemostasis and no intraoperative deaths were encountered (30-day mortality: 0%). Postoperative complications occurred in 84 patients (30-day morbidity rate, 10.3%). Major complications included prolonged air leakage (6.2%), mild arrhythmia (2.2%), bacterial pneumonia (1.6%), acute exacerbation of interstitial lung diseases (0.9%), and pulmonary embolism (0.2%).
Full table
Full table
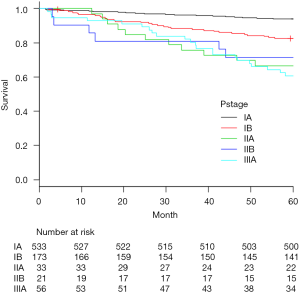
The most common histopathologic type was adenocarcinoma (75.6%). Among those cases, 91 patients (11%) showed multiple synchronous primary cancers. Median number of dissected lymph nodes was 19 (range, 5–53). Pathological upstaging was noted in 189 patients (23%), comprising nodal upstaging in 103 patients (12.6%), mediastinal upstaging (cN0 to pN2) in 54 patients (6.6%), and hilar or peribronchial upstaging (cN0 to pN1) in 49 patients (6.0%). In accordance with the protocol at our center, postoperative chemotherapy was administered to all patients except those who showed pathologic stage IA. A total of 116 deaths were registered: 73 patients died of disease progression, and 43 of other causes. The 5-year OS rate of the 816 patients was 89.2%. Five-year OS rates were 90.2% in clinical stage IA patients and 85.2% in IB patients. For patients in pathologic stage IA, IB, IIA, IIB and IIIA, 5-year OS rates were 94.2%, 86.1%, 69.7%, 81.0% and 66.1%, respectively (Figure 2).
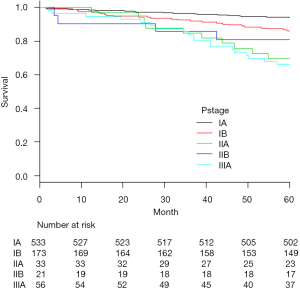
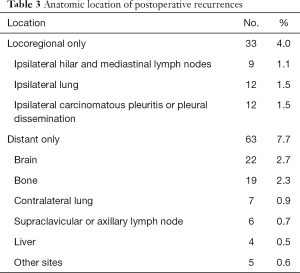
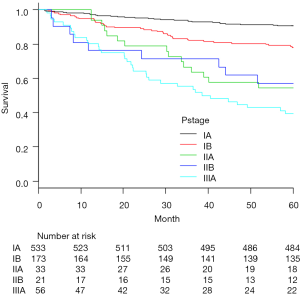
Recurrence was documented in 114 patients (14.0%) and classified as follows: locoregional only in 33 cases (4.0%), distant only in 63 (7.7%) and locoregional + distant in 18 (2.2%) (Table 3). Median time from surgery to locoregional recurrence was 24.0 months (range, 3–64 months). Median time to distant recurrence was 24.2 months (range, 3–75 months). Of the 33 identified locoregional recurrences, 9 recurred within ipsilateral hilar and mediastinal lymph nodes, 12 in the ipsilateral lung and 12 in ipsilateral carcinomatous pleuritis or pleural dissemination. Of the 9 cases, diagnosis was pathologic N0 in 3 cases, N1 in 4, and N2 in 2. Histopathologic type was papillary adenocarcinoma in 4 of these 8 patients (50%). Recurrent lymph nodes were included within the area of selective node dissection in 5 cases, and were outside the area of systematic mediastinal lymphadenectomy in 4 cases. Of the 12 cases with ipsilateral carcinomatous pleuritis or pleural dissemination, PL3 visceral pleural invasion was diagnosed in 1 case, PL2 in 4, PL1 in 1 and PL0 in 6. Among these, 7 patients (58.3%) were diagnosed pre- or intraoperatively by needle biopsy. No port-site recurrences were identified. The 5-year actuarial locoregional recurrence-free survival rate was 87.6%, and the 5-year DFS rate was 82.2%. For patients in pathologic stage IA, IB, IIA, IIB and IIIA, corresponding 5-year locoregional recurrence-free and DFS rates were 94.0% and 90.8%, 82.6% and 78.0%, 66.7% and 54.5%, 71.4% and 57.1%, and 60.7% and 39.3%, respectively (Figures 3,4).
Full table
Discussion
Lung cancer is the most frequent cause of cancer-related deaths worldwide. In cases of primary early-stage lung cancer, surgical resection with VATS represents the gold standard of treatment options with curative intent (9). However, despite using VATS for more than 20 years, consensus has yet to be reached on clear definitions. Various attempts at clarifying the terminology have been published. Migliore et al. suggested the term VATS for surgery using a single skin incision of 5–6 cm and 2–4 ports, where the camera is separated from the other instruments (10). An annual report by the Japanese Association for Thoracic Surgery defined VATS as a surgical procedure using a skin incision >8 cm long and/or a minithoracotomy (hybrid) approach (1). The Cancer and Leukemia Group B (CALGB) 39802 trial in 2007 established a definition of VATS lobectomy that included: no use of rib spreading; an incision £8 cm in length to deliver specimens; and individual dissection of veins, arteries and airway combined with standard lymph node sampling or dissection (11). The CALGB definition is presumably the most accepted. Furthermore, efforts have been made over the last 20 years to minimize the size and number of ports to achieve maximal benefits in patient treatment. Recent technical innovations include uniportal VATS (12) and robot-assisted thoracic surgery (RATS) (13). Given this background, concerns regarding oncologic efficacy and safety have limited the acceptance of VATS procedures. This single-institute, retrospective study evaluated the surgical and long-term oncological outcomes of patients with NSCLC who underwent 3-port VATS lobectomy with selective lymphadenectomy.
The advantages of lobectomy by VATS compared to thoracotomy in regard to postoperative morbidity and mortality represent the most indisputable issue. Many surveys, including meta-analyses and RCTs, have covered this topic (2-7). Long et al. reported an RCT in which VATS (215 patients) was compared with axillary thoracotomy (210 patients) for clinically early-stage NSCLC in terms of short-term and oncologic efficacy (14). Morbidity rates were 10.2% and 10.9%, respectively (as compared with 10.2% in the present study), and no operative mortality was documented for the whole cohort (mirroring the 0% in the present study). Median operative times were 150 and 166 min (compared to 185 min in the present study), while blood loss was 100 and 100 mL (and also 100 mL in the present study), and the rate of conversion to thoracotomy was 3.7% (0.3% in this study), and postoperative hospital stay was 14 and 15 days (6 days in this study). The present results indicate that VATS lobectomy is safe and efficacious, with results comparable to those of thoracotomy.
Despite numerous reports on VATS lobectomy for NSCLC, locoregional recurrence after VATS has rarely been discussed. Haruki et al. reported locoregional recurrence after VATS lobectomy or segmentectomy with mediastinal lymph node dissection for primary lung cancer (15). The results showed 47 cases of postoperative recurrence among the 248 included patients. These recurrences were classified as locoregional only in 15 cases (6.0%), distant only in 26 (10.5%), and both locoregional and distant in 6 (2.4%). Of the 15 locoregional only cases, 2 involved the surgical margin (0.4%), 5 involved the ipsilateral pleura (2.0%), and 8 recurred within ipsilateral hilar and mediastinal lymph nodes (3.2%). With our VATS lobectomy technique using selective lymphadenectomy for clinical stage I NSCLC, locoregional recurrence only was identified in 33 cases (4.0%) and the 5-year locoregional recurrence-free survival rate was 87.6%. Among our 33 cases with locoregional recurrence only, 12 concerned the ipsilateral pleura (1.5%), 9 recurred within ipsilateral hilar and mediastinal lymph nodes (1.1%) and 12 arose in the ipsilateral lung (1.5%). Lymph node recurrence developed in 4 patients in an area from which mediastinal lymphadenectomy had been omitted. Of those 4 patients, 2 patients were pathologic stage I with recurrence to subcarinal lymph nodes. These cancers were located close to a lobe fissure (right S2 and left S1+2) and were supposed to had undergone systematic mediastinal node dissection. Systematic lymphadenectomy might have resulted in better prognosis. Of the 12 patients with ipsilateral carcinomatous pleuritis or pleural dissemination, 7 patients (58.3%) were diagnosed pre- or intraoperatively by needle biopsy (2 patients diagnosed by CT-guided needle biopsy and 5 patients diagnosed by intraoperative needle biopsy). Among them, 4 cases were without pleural invasion (PL0). The relationship between these disseminations and the form of needle biopsy remains unclear. Some reports have suggested that tumor dissemination along the needle tract is a rare complication, with an overall reported incidence <1% (16). However, our results call attention to the potential risks of needle biopsy and suggest the need for further investigations focusing on pleural recurrence after needle biopsy.
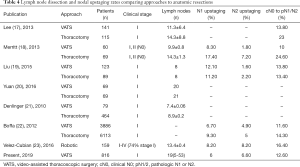
Adequate lymph node dissection is an essential part of the surgical management of NSCLC, facilitating the assessment of prognosis and determining therapeutic options. Two important values when comparing VATS to thoracotomy are the number of lymph nodes harvested and the rate of nodal upstaging. Pathologic nodal upstaging can be used as a proxy for the completeness of nodal evaluation. Early studies examining approaches showed that thoracotomy retrieved more lymph nodes and offered a higher rate of nodal upstaging as compared to VATS (Table 4) (17-23). Lee et al. reported that compared to VATS, thoracotomy yielded more nodes (14.3 vs. 11.3, respectively) and removed more nodal stations (3.8 vs. 3.1, respectively) (17). Interestingly, they found no differences in 3- or 5-year OS or DFS rates between groups (e.g., 3- and 5-year OS rates of 87.4% and 76.5% for VATS, and 81.6% and 77.5% for thoracotomy, respectively). Merritt et al. reviewed their institutional data comparing thoracotomy to VATS for cN0 NSCLC and found that the thoracotomy group showed dissection of significantly more lymph nodes (14.6 vs. 9.9 nodes, respectively) (18). They also found a higher rate of nodal upstaging to N1/N2 (24.6% vs. 10%, respectively). Conversely, Liu et al. reviewed 212 consecutive lobectomies at their institution to compare VATS and thoracotomy (19). They reported comparable numbers of removed lymph nodes, with nodal upstaging rates of 13.8% for VATS and 13.4% for thoracotomy lobectomy. No differences were identified in 3- or 5-year OS or DFS rates between these approaches. Yuan et al. analyzed 138 patients with clinical stage I disease, divided into propensity-matched groups, who underwent lobectomy under either VATS or thoracotomy (20). The number of dissected lymph nodes was similar (20 nodes for VATS vs. 21 nodes for thoracotomy). In the present study, the number of dissected lymph nodes (19 nodes) and the rate of nodal upstaging (total 12.6%) were comparable to or superior to values from those reports. Five-year DFS and OS rates were acceptable compared with rates reported in previous studies (Table 5) (17,19,20).
Full table

Full table
Since the first descriptions of VATS for pulmonary resections in patients with malignant lung tumors, procedures have been gradually but substantially refined. The number of ports and the diameters of the camera and other instruments have decreased minimize the invasiveness of the surgical approach and provide increasingly less trauma. Recent results of this evolutionary process are uniportal VATS and RATS. However, due to the lack of RCTs comparing uniportal VATS with thoracotomy or multiport VATS, the feasibility and safety of uniportal VATS have only been shown in retrospective and observational studies so far (24). Compared against thoracotomy, uniportal VATS seems to offer superior perioperative results. A retrospective study comparing long-term results after VATS, RATS and open surgery was published by Kwon et al. (25). The 3 groups were matched by propensity scoring, and showed no significant differences in OS between groups (VATS vs. RATS, P=0.10; Open vs. RATS, P=0.53; VATS vs. Open, P=0.08). In our department, we began using needlescopic-assisted thoracoscopic surgery in 2015, anticipating that the favorable postoperative outcomes associated with 3-port VATS would be retained. The quintessence of surgery for lung cancer must remain oncological curability.
In conclusion, our report suggests that 3-port VATS lobectomy with mediastinal lymphadenectomy represents a feasible approach for primary lung cancer, offering long-term outcomes equivalent or superior to those of conventional approaches. This is regardless of whether multi portal VATS, uniportal VATS or RATS is used in the individual institution. In the recent literature, each of these minimally invasive surgical approaches have shown similar results in terms of OS, rates of major postoperative complications and morbidity, and duration of hospital stay.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mingyon Mun) for the series “Oncological Clearance of VATS Lobectomy for Clinical N0 Non-small Cell Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2019.12.05). The series “Oncological Clearance of VATS Lobectomy for Clinical N0 Non-small Cell Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Clinical Research at Toranomon Hospital (approval No. 1179). The need to obtain written informed consent from each patient was waived because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shimizu H, Endo S, Natsugoe S, et al. Thoracic and cardiovascular surgery in Japan during 2016. Gen Thorac Cardiovasc Surg 2019;67:377-411. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Gonfiotti A, Bertani A, Nosotti M, et al. Safety of lymphadenectomy during video-assisted thoracic surgery lobectomy: analysis from a national database. Eur J Cardiothorac Surg 2018;54:664-70. [Crossref] [PubMed]
- Dziedzic R, Marjanski T, Binczyk F, et al. Favourable outcomes in patients with early-stage non-small-cell lung cancer operated on by video-assisted thoracoscopic surgery: a propensity score-matched analysis. Eur J Cardiothorac Surg 2018;54:547-53. [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Zhang Z, Zhang Y, Feng H, et al. Is video-assisted thoracic surgery lobectomy better than thoracotomy for early-stage non-small-cell lung cancer? A systematic review and meta-analysis. Eur J Cardiothorac Surg 2013;44:407-14. [Crossref] [PubMed]
- Bendixen M, Jorgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Sobin LH, Gospodrowicz MK, Wittekind CH. TNM Classification of malignant tumors, 7thed. New York, NY: Wiley-Blackwell; 2009.
- NCCN Clinical Practice Guidelines in Oncology. Non-Small-Cell Lung Cancer. Version 6 2017.
- Migliore M, Deodato G. Thoracoscopic surgery, video-thoracoscopic surgery, or VATS: a confusion in definition. Ann Thorac Surg 2000;69:1990-1. [Crossref] [PubMed]
- Swanson SJ, Herndon JE II, D’Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Sihoe AD. Uniportal video-assisted thoracic (VATS) lobectomy. Ann Cardiothorac Surg 2016;5:133-44. [Crossref] [PubMed]
- Wei S, Chen M, Chen N, et al. Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: a systematic review and meta-analysis. World J Surg Oncol 2017;15:98. [Crossref] [PubMed]
- Long H, Tan Q, Luo Q, et al. Thoracoscopic surgery versus thoracotomy for lung cancer: short-term outcomes of a randomized trial. Ann Thorac Surg 2018;105:386-92. [Crossref] [PubMed]
- Haruki T, Miwa K, Araki K, et al. Distribution and prevalence of locoregional recurrence after video-assisted thoracoscopic surgery for primary lung cancer. Thorac cardiovasc Surg 2016;64:526-32. [PubMed]
- Seyfer AE, Walsh DS, Graeber GM, et al. Chest wall implantation of lung cancer after thin-needle aspiration biopsy Ann Thorac Surg 1989;48:284-6. [Crossref] [PubMed]
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951-60. [Crossref] [PubMed]
- Merritt RE, Hoang CD, Shrager JB. Lymph node evaluation achieved by open lobectomy compared with thoracoscopic lobectomy for N0 lung cancer. Ann Thorac Surg 2013;96:1171-7. [Crossref] [PubMed]
- Liu C, Li Z, Bai C, et al. Video-assisted thoracoscopic surgery and thoracotomy during lobectomy for clinical stage I non-small-cell lung cancer have equivalent oncological outcomes: A single-center experience of 212 consecutive resections. Oncol Lett 2015;9:1364-72. [Crossref] [PubMed]
- Yuan J, Dai G, Kong F. Long-term outcomes of video assisted thoracoscopic versus open lobectomy for nonsmall-cell lung cancer with propensity score matching. Int J Clin Exp Med 2016;9:3572-8.
- Denlinger CE, Fernandez F, Meyers BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg 2010;89:1730-5. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53. [Crossref] [PubMed]
- Velez-Cubian FO, Rodriguez KL, Thau MR, et al. Efficacy of lymph node dissection during robotic assisted lobectomy for non-small cell lung cancer: retrospective review of 159 consecutive cases. J Thorac Dis 2016;8:2454-63. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Kwon ST, Zhao L, Reddy RM, et al. Evaluation of acute and chronic pain outcomes after robotic, video assisted thoracoscopic surgery, or open anatomic pulmonary resection. J Thorac Cardiovasc Surg 2017;154:652-659.e1. [Crossref] [PubMed]
Cite this article as: Suzuki S, Fujimori S, Kohno T, Nagano M, Kikunaga S. Long-term outcomes of 3-port VATS lobectomy with selective mediastinal lymphadenectomy for clinical stage I non-small cell lung cancer. Video-assist Thorac Surg 2020;5:2.