
Tips for uniportal video assisted thoracic surgery S1 segmentectomy
Introduction
Anatomic segmentectomies are playing an important role in thoracic surgery as a result of the increased implementation of screening programs for lung cancer which have lead surgeons to face lesions in earlier stages more often (1,2). Segmentectomy has become an option for the management of small lesions allowing anatomical pulmonary resections to be used both for diagnosis, in cases not suitable for percutaneous lung biopsy, as well as for the treatment of lesions while sparing lung parenchyma (3).
The currently accepted indication are ground glass opacity (GGO) lesions with a diameter lesser than 2 cm in a peripheral location in context of lung cancer suspected diagnosis (cT1aN0M0) for selected patients (4), benign lesions, and pulmonary metastases.
The technique has evolved from open thoracotomy, to a hybrid VATS technique adapted by Nomori et al. (5), later, Rocco et al. implemented it through VATS (6), and lately Gonzalez-Rivas et al. developed the UniVATS approach (7). Our group started its UniVATS practice in 2014; afterwards this paper’s author contributed to Xie et al. in the largest series of segmentectomies by UniVATS approach published in 2016 (8).
Regarding the technique itself, a complete resection of the affected segment is essential while treating lung cancer because it could have an impact in the long-term results. During its evolution, the open technique’s steps were adapted to keep its oncological principles. Different possible solutions came up for this objective, as the way of dissecting segmental structures or intersegmental margin delimitation and division. We detail our approach and technique for UniVATS S1 segmentectomies.
Patient selection
The patient selection shares indications and contraindications for every UniVATS procedure. It also shares the indications already mentioned for segmentectomies such as less than 2 cm GGO lesions (T1a N0 M0). In the context of lung cancer patients, it is important to rule out N2 disease or calcified lymph nodes before the operation.
Preoperative preparation
The patient’s management is approach in a “fast-track” strategy since the preoperative preparation using an enhanced recovery after surgery (ERAS) protocol. This could have an impact on the patient’s recovery by minimizing the stress response to surgery, with a possible repercussion on the outcomes, length of stay, and costs (9,10).
We use percutaneous localization of the lesions when suitable. A hook-wire is placed in the lesion before the operation.
Equipment and set up
Patient’s position
The patient is routinely managed under general anesthesia with one-lung ventilation, although in selected cases we choose a non-intubated anesthesia approach.
The patient is positioned in a lateral decubitus position according to the side of the lesion to be operated. When using the subxiphoid approach, a 45 degrees intermediate lateral decubitus is chosen.
Set up
A secondary instruments table is placed over the operating table at the patient’s feet. The scrub nurse and the main instruments table are located at the right side of the operating table beside the secondary instruments table. The surgeon will place himself in front of the patient and the assistant will be positioned at the patient’s back, beside the scrub nurse.
The screen, camera and light source are placed at the head of the operating table. The use of a FullHD or UltraHD camera is important as it allows clearer distinction of structures to be dissected or transected. The endoscope used is a 30 degrees, 10 mm thoracoscope.
A set of UniVATS double jointed instruments is used. In case we use the subxiphoid approach, a subxiphoid UniVATS instruments set is essential because they are longer and they have a different angled curve than those designed only for UniVATS intercostal approach. This way the surgeon is able to reach any intra-thoracic structure and aspect. We also use advanced energy devices for dissection and vessel division.
Surgical technique for UniVATS S1 segmentectomy
UniVATS approach
An incision smaller than 4 cm is placed between the middle and anterior axillary line on the 5th intercostal space. For the right upper lobe approach, the 4th intercostal space is suggested. Some surgeons prefer the 4th intercostal space also for upper lobes segmentectomies.
The Serratus anterior muscle fibers should be opened without cutting them in a muscle-sparing way.
The intercostal muscles should be divided along the upper edge of the inferior rib, trying not to divide it more than necessary to decrease the trauma to the intercostal space. After entering the pleural cavity, a wound retractor is placed.
Subxiphoid UniVATS approach
A 3 to 4 cm incision is performed in a longitudinal median plane from the xipho-sternal junction to 1cm below the xiphoid process. We resect the process and then we use a combination of blunt and energy retrosternal dissection towards the hemithorax to be approached until entering the pleural cavity. A part of the mediastinal fat pad is resected and a wound retractor is placed.
S1 segmentectomy technique
It is essential to meticulously review the patient’s anatomy by all the available means to be sure the lesion’s location, identification of anatomical structures, and to define the strategy. In the following description, anatomical structures are named by nomenclature previously described by Nomori et al. (11).
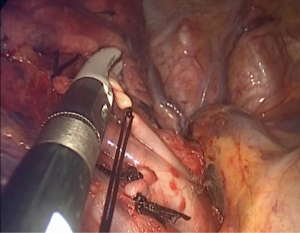
The Lung is retracted posteriorly and slightly inferiorly by using a lung grasper, this traction will expose the superior pulmonary vein in the hilum. We dissect the hilum using hook, cautery or energy devices from the ventral aspect to a dorsal direction identifying the different hilar structures. The segment structures are identified and then carefully dissected with a combination of blunt and energy dissection, this step will make the procedure safer and faster. To divide the vessels we prefer to tide their proximal portion using a 3.0 silk suture, and later we divide the distal portion by an advanced energy devices (Figure 1). This way we deal firstly with the V1 vein and then we approach the A1 artery (Figure 2).

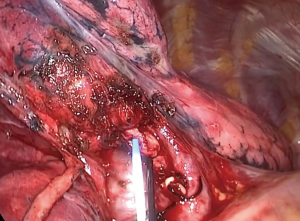
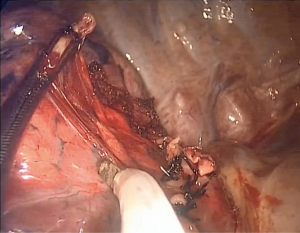
After dividing the vessels, the bronchus is dissected and tied proximally and then is partially cut open distally by scissors. Afterwards, a central vein catheter is inserted into the distal segmental bronchus and air is insufflated defining the intersegmental plane (Figure 3), in a technique reported previously by the author (13) which we detail further in this paper. Thereafter, the bronchus is divided by stapler, the distal bronchial stump is lifted and dissection is performed by cautery along the bronchus, dividing part of the intersegmental plane and some branches of the affected segment’s drainage vein, with no special dissection for intersegmenal veins (Figure 4). This dissection will leave a space to be used later for the stapler positioning while dividing the rest of the intersegmental plane, potentially achieving its more accurate division by helping to compress less lung parenchyma belonging to neighboring segments in the stapling line, also promoting a better future insufflation of the remaining segments.
Once the procedure is concluded, we check for air leakage, hemostasis and a 28 Fr chest drain is placed at the posterior part of the incision.
Right S1 segmentectomy highlights
After identifying V1 and the central vein, we dissect the structure and we transect it. By dividing V1 better access is achieved for dissecting A1. At this point the recurrent A2 must be identified and preserved, A1 runs towards the B1 bronchus, contrary to a recurrent A2 that goes apart from it. Then if A2 is not clearly identified, the first dorsal branch from A1 must be preserved because it might be the recurrent A2 (11). Afterwards the B1 is identified, exposed and divided after the delimitation of the segment (Figure 2). Finally the intersegmental borders are divided by staplers.
Left S1 segmentectomy highlights
Anatomical differences determine the S1+2 segment in the left lung. Anyway, the left S1 portion can be resected separately when the anatomy is clear by an atypical segmentectomy. After dissecting V1+2 a, a superior branch of A1+2 (A1+2 a+b) can be identified and divided. Afterwards, the identification and dissection of B1+2 a+b is possible (Figure 5). We repeat the process before detailed for the segmentectomy completion.

Postoperative management
Normally, the patients are extubated in the OR and transferred to a post anesthesia care unit (PACU) where they will be monitored for a short period of time of at maximum 2 hours. After that period the patients will be transferred to the regular ward or the ICU, according to the specific criteria.
If the patients remain stable during their stay at the ward, the first X-ray will be done during the next morning. The pain management protocol includes patient control analgesia (PCA) and oral analgesics if needed.
Indications for removing the chest tube, are the same as in the open thoracotomy technique.
The attachment to the ERAS strategy including early mobilization, effective analgesia, pulmonary rehabilitation and appropriate drain management could be helpful (9,10).
Tips tricks and pitfalls
Tide instead of clip
We choose tying rather than using anti-slip clips for small vessels, because the use of clips would result in a “mined-field”, creating obstruction for subsequent complex maneuvers such as stapling or those needed to divide the intersegmental plane. After tying the proximal portion of the vessel, we use an advanced energy device to transect it distally to the tie (Figure 1).
Lifted bronchus dissection
As in the classic open technique, we lift the distal bronchial stump and dissect the parenchyma in its posterior aspect (Figure 4). We avoid dissecting the intersegmental veins distally, which is time consuming and difficult. By dividing part of the intersegmental plane we gain space for maneuvering thus decreasing the risk of damaging surrounding structures (Figure 6); Additionally this technique creates a space to put the anvil of the stapler while dividing the delimited intersegmental border after one of the insufflation techniques; This will provide a more anatomical resection, with a possible impact on the long-term results and on the remaining lung’s functionality.

Open insufflation technique
Currently we lack an efficient method to demarcate the segments to be removed. Our technique is based on the selective insufflation of the excluded segment in a safe, less time consuming and well-balanced cost-effective way. The elements needed for this method are a central venous catheter connected to a 60 mL Luer-slip syringe.
Once the bronchus is exposed, a 3.0 silk suture tie is placed at its proximal portion. Next, the bronchus is partially cut distally to the tie in its anterior aspect. This will allow the tip of the central vein catheter to be inserted into its lumen (Figure 3). Following this, air is blown from the syringe insufflating the affected segment until the demarcation intersegmental line is noticed (Figure 7).

With this method we notice advantages such as a better demarcation line than in the inflation-deflation technique, possibly due to less collateral inflation taking place, potentially achieving more accurate margin’s resection. Another advantage is the time invested in this procedure, avoiding waiting 15 minutes for the lung to deflate. Furthermore, no special or expensive equipment is needed (bronchoscope, high-frequency oscillation device, or near-infrared imaging systems); Also these procedure’s specific complications are avoided. It is a feasible, and we believe more effective method to approach this step.
A possible pitfall could be that the partial cut in the segmental bronchus and the insertion of the catheter could be difficult for inexperienced surgeons.
Conclusions
Segmentectomies are playing a growing role in thoracic surgery. Along the evolution of their technique, efforts have been invested in order to achieve a less invasive technique while respecting the oncological principles. We believe that our approach achieves these goals in a more efficient way. We still must fulfill the scientific scrutiny that could demonstrate its benefits compared to other techniques and to assess its long-term results.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Hitoshi Igai) for the series “Uniportal VATS Segmentectomy” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2020.01.06). The series “Uniportal VATS Segmentectomy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Jacobson FL, Austin JH, Field JK, et al. Development of The American Association for Thoracic Surgery guidelines for low-dose computed tomography scans to screen for lung cancer in North America: recommendations of The American Association for Thoracic Surgery task force for lung cancer screening and surveillance. J Thorac Cardiovasc Surg 2012;144:25-32. [Crossref] [PubMed]
- Takashima S, Maruyama Y, Hasegawa M, et al. CT findings and progression of small peripheral lung neoplasms having a replacement growth pattern. AJR Am J Roentgenol 2003;180:817-26. [Crossref] [PubMed]
- Sepesi B, Walsh GL. Surgical therapy of ground-glass opacities. Semin Diagn Pathol 2014;31:289-92. [Crossref] [PubMed]
- Nomori H, Okada M. General knack of segmentectomy. In: Nomori H, Okada M, editors. Illustrated anatomical segmentectomy for lung cancer. 1st ed. Tokyo: Springer, 2012:9-21.
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Mendez L, et al. Single-port video-assisted thoracoscopic anatomic segmentectomy and right upper lobectomy. Eur J Cardiothorac Surg 2012;42:e169-71. [Crossref] [PubMed]
- Xie D, Wang H, Fei K, et al. Single-port video-assisted thoracic surgery in 1063 cases: a single-institution experience. Eur J Cardiothorac Surg 2016;49:i31-6. [Crossref] [PubMed]
- Dinic VD, Stojanovic MD, Markovic D, et al. Enhanced Recovery in Thoracic Surgery: A Review. Front Med (Lausanne) 2018;5:14. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Nomori H, Okada M. Illustrated anatomical segmentectomy for lung cancer. Tokyo: Springer, 2012.
- Bulgarelli Maqueda L, Jiang L. Video demonstrating the dissection steps during a right S1 subxiphoid UniVATS segmentectomy. Asvide 2020;7:054. Available online: http://www.asvide.com/watch/33094
- Soultanis CM, Chenchao M, Lei J. Open insufflation: a novel technique for identifying the intersegmental border. Asian Cardiovasc Thorac Ann 2019;27:138-40. [Crossref] [PubMed]
- Bulgarelli Maqueda L, Jiang L. Video demonstrating the dissection steps during a left S1 subxiphoid UniVATS segmentectomy. Asvide 2020;7:055. Available online: http://www.asvide.com/watch/33095
- Bulgarelli Maqueda L, Jiang L. Video playing the dissection of part of the intersegmental plane, performed after the distal bronchial stump is lifted, during a right S1 UniVATS segmentectomy. Asvide 2020;7:056. Available online: http://www.asvide.com/watch/33096
- Bulgarelli Maqueda L, Jiang L. Video presenting the open insufflation technique during a S3 subxiphoid UniVATS segmentectomy. Asvide 2020;7:057. Available online: http://www.asvide.com/watch/33097
Cite this article as: Bulgarelli Maqueda L, Jiang L. Tips for uniportal video assisted thoracic surgery S1 segmentectomy. Video-assist Thorac Surg 2020;5:8.


