
Surgery for simultaneous bilateral primary spontaneous pneumothorax: when and how?
Introduction
Primary spontaneous pneumothorax (PSP) is a common benign disease affecting adolescents and young adults. In Taiwan, a national population-based cohort study in 2013 documented an incidence of 7.18/100,000 (1), which is comparable with the data (1.2 to 18/100,000) reported in Western countries (2). However, simultaneous bilateral primary spontaneous pneumothorax (SBPSP) is a relatively rare clinical event with limited data on its incidence in the literature (3). Its clinical symptoms and signs vary and include chest pain, mild shortness of breath, and even tension pneumothorax (4). Patients with SBPSP may deteriorate rapidly if left untreated. Therefore, early and prompt management is recommended when SBPSP is recognized. Although surgery is advocated for SBPSP, based on international consensus mentioned in the leading guidelines (2,5), relevant studies of SBPSP are scarce and reported mostly as cases. The aim of this article is to provide a literature review of SBPSP, mainly focused on its epidemiology, diagnosis, management strategy, and future perspectives.
Epidemiology
The incidence of simultaneous bilateral spontaneous pneumothorax (SBSP) has been reported to be around 7.8–20% of all cases of spontaneous pneumothorax (6). Studies by Sayar et al. and Akcam et al. reported that approximately 58% and 68% of patients with SBSP, respectively, had causative underlying pulmonary disease (3,7). Several medical conditions known to be associated with SBSP include lung metastasis, tuberculosis, histocytosis X, and COPD (3). SBSP in a patient without an underlying medical condition is regarded as SBPSP, shown to account for 1.6% of first presentation in PSP patients in one large cohort study (6). In other large case series, SBPSP constitutes approximately 2-2.4% of patients operated for PSP (8,9).
Pathophysiology
In PSP, air leaks through the visceral pleura, possibly induced by a rapid increase in transpulmonary pressure or defects of visceral pleura (10). While the exact pathogenesis of PSP is not completely understood, rupture of blebs/bullae that cause air to leak into the pleural space remains the significant factor explaining the occurrence of PSP. In fact, apical blebs/bullae are found in the majority of PSP patients during video-assisted thoracic surgery (VATS) and in nearly all patients during thoracotomy (11,12). These subpleural air-containing lesions are associated with other factors such as patient height, distal airway inflammation, hereditary predisposition, low body mass index, which may predispose to PSP (13). Additionally, some authors reported that patients with SBPSP tend to be younger, with lower body mass index, and have abnormal blebs/bullae (6,14).
Moreover, in extremely rare circumstances, SBPSP with congenital pleuro-pleural communication has been reported (15,16). The reported sites of congenital pleuro-pleural communication were the anterior mediastinum and the lower middle mediastinum. Therefore, the aforementioned mediastinal communication should be carefully inspected during surgery for SBPSP, and closure of the pleuro-pleural communication is essential to prevent future recurrence (16).
Clinical manifestation and diagnosis
SBPSP, which is similar to PSP, occurs most often at rest. A typical physical examination tends to reveal decreased breath sounds and reduced chest expansion bilaterally. Although hemodynamic compromise or significant hypoxemia is uncommon in PSP, the simultaneous occurrence of PSP carries potentially more severe clinical symptoms such as chest pain, shortness of breath, and even tension pneumothorax (4). In light of the variability of presented clinical features, the diagnosis of SBPSP is often confirmed with radiographic imaging such as thoracic radiography or computed tomography. Since rupture of the blebs in the lung is the most common cause of PSP, the presence of these air-containing lesions on high-resolution computed tomography (HRCT) has been regarded as a significant predictor for PSP recurrence (17). The reported incidence of blebs/bullae on HRCT ranged from 47% to 94% in ipsilateral lung (17-23) and 55% to 70% in contralateral lung (21-23). Further, Lee et al. mentioned that patients with SBPSP had a significantly higher incidence of blebs/bullae observed on HRCT compared with non-SBPSP (88.5% vs. 63.5%) (6). Therefore, in patients of SBPSP potentially requiring surgery, HRCT may be useful to identify more occult pulmonary lesions such as blebs/bullae, as well as guiding surgical management.
Management
According to the British Thoracic Society (BTS) guidelines and American College of Chest Physicians (ACCP) Delphi consensus statement (2,5), SBPSP remains one of the indications for definitive treatment of PSP, such as pleurodesis or surgery. Nevertheless, no clear decision strategy regarding SBPSP has been established, possibly due to the scarcity of such case events.
In order to obtain the relevant treatment experiences for SBPSP, we identified case series over the last three decades in the literature (excluding single case report and studies with incomplete clinical feature and outcome) (shown in Table 1) (6-9,24-27). The following are the associated treatment strategies extracted from the literature with regards to treatment of SBPSP.
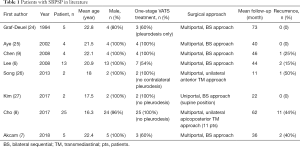
Full table
Chest drainage
Based on the aforementioned guidelines, supplemental oxygen therapy, percutaneous needle aspiration, or a more aggressive procedure like intercostal drain placement are recommended, depending on the clinical symptoms and the size of the pneumothorax (2,5). Following the same suggestions, unilateral or bilateral chest drainage should be administered for patients of SBPSP involving larger or symptomatic pneumothorax. For those PSP patients with mild-to-moderate extent of simultaneous occurrence, surgical intervention can be offered directly if not physiologically contraindicated (8). However, from a practical point of view, it could be difficult to make therapeutic recommendations according to these guidelines in developing countries with restricted facilities. Instead of VATS treatment, Flores-Franco et al. reported their successful experience using bilateral catheter drainage with Heimlich valves for one patient of SBPSP. They considered that this alternative approach could be useful in places with limited resources for VATS (28).
One-stage operation
With the advances in minimally invasive techniques, bilateral surgery for pneumothorax via conventional approach including sternotomy or thoracotomy was replaced by VATS in the early 2000s (24,25,29). Nowadays, one-stage bilateral VATS has been advocated as a standard method in managing patients with ipsilateral PSP and contralateral blebs/bullae (21,22,30,31). In the relevant literature regarding surgery for SBPSP, similarly, one-stage VATS treatment has been considered the preferable choice through multiportal and bilateral sequential (BS) approach (6,7,9,24,25,27).
Uniportal VATS
In recent decades, since uniportal VATS has gained increasing feasibility and popularity, and its employment in the treatment of PSP and bilateral pulmonary lesions has been reported to be effective (32,33). In 2017, Kim used the one-stage uniportal VATS technique in the supine position to treat SBPSP patients, and succeeded in achieving less operative time and better cosmetics (27).
Unilateral trans-mediastinal approach
Although one-stage VATS using BS approach has been mostly undertaken for treatment of SBPSP, it is associated with chronic incisional pain, poor anesthetic outcomes, and increased operative time. In the literature, Wu et al. reported the very first study treating ipsilateral PSP with contralateral bullae using anterior transmediastinal (TM) approach through unilateral incision (34). Later, several reports discussed this unilateral TM approach, which can also be performed in SBPSP, and showed its efficacy and low morbidity (8,26). Despite the favorable outcomes brought by this TM approach, including decreased operative time, duration of drainage, hospital stay, and potential cosmetic effect, this procedure has certain contraindications, such as a history of sternotomy and intolerance to single-lung ventilation. Notably, the authors also claimed that, in their experience, VATS bullectomy through this unilateral TM approach is more suitably performed on the right side.
Subxiphoid approach
Moreover, in pursuit of reducing acute and chronic postoperative wound pain, subxiphoid VATS has been adopted in a wide array of thoracic operations, including major lung resections (35,36). However, subxiphoid approach for SBPSP is not mentioned in the literature. To date, subxiphoid approach for patients of ipsilateral PSP with contralateral blebs/bullae only has been reported in limited studies (37,38). Their results showed that significantly lower postoperative pain scores and longer operative time were observed in the subxiphoid VATS group compared with the transthoracic VATS group. Interestingly, perioperative data including duration of drainage and hospital stay did not differ significantly.
Summary
Although SBPSP is rare and its clinical features variable, ranging from mild dyspnea to tension pneumothorax. Based on guideline recommendations, once early diagnosis is established and/or urgent chest drainage is administered, such SBPSP patients who are inherently young and physiologically fit are considered suitable to undergo one-stage VATS treatment.
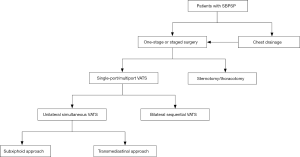
The current trend in VATS is to utilize fewer ports and less invasive access to reduce postoperative wound pain, chronic wound paresthesia, operative time, duration of drainage, and hospital stay. Even though the aforementioned innovative VATS techniques using TM or subxiphoid approach have demonstrated satisfactory results for simultaneous resection of bilateral pulmonary bleb/bullae, the long-term outcomes including recurrence rate have not been validated due to the limited number of cases and studies. For example, we know that the role of pleurodesis is essential in the prevention of postoperative recurrence after VATS bullectomy. Because contralateral pleurodesis cannot be efficiently performed through the unilateral TM approach, this may have contributed to the high recurrence rate (44%) in the largest case series of SBPSP to date (8). In addition, subxiphoid VATS may potentially be an alternative way to manage patients with SBPSP in selected conditions, but this necessitates further clinical studies to verify its efficacy and feasibility. An algorithm for the surgical approach in SBPSP has been proposed and is demonstrated in Figure 1. In conclusion, all the above-mentioned novel techniques should be used appropriately in carefully selected patients. Surgical access should be safe and easily reproducible in order to be employed by thoracic surgeons on a routine basis. Otherwise, one-stage VATS using BS approach remains the reliable and effective procedure in the treatment of SBPSP.
Acknowledgments
The authors thank the help of Jadzia Chou on language revision.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jin-Shing Chen, Ke-Cheng Chen and Mong-Wei Lin) for the series “VATS: Primary Spontaneous Pneumothorax” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2019.09.03). The series “VATS: Primary Spontaneous Pneumothorax” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huang YH, Chang PY, Wong KS, et al. An Age-Stratified Longitudinal Study of Primary Spontaneous Pneumothorax. J Adolesc Health 2017;61:527-32. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii18-31. [Crossref] [PubMed]
- Sayar A, Turna A, Metin M, et al. Simultaneous bilateral spontaneous pneumothorax report of 12 cases and review of the literature. Acta Chir Belg 2004;104:572-6. [Crossref] [PubMed]
- Rim T, Bae JS, Yuk YS. Life-threatening simultaneous bilateral spontaneous tension pneumothorax - A case report. Korean J Thorac Cardiovasc Surg 2011;44:253-6. [Crossref] [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [Crossref] [PubMed]
- Lee SC, Cheng YL, Huang CW, et al. Simultaneous bilateral primary spontaneous pneumothorax. Respirology 2008;13:145-8. [PubMed]
- Akcam TI, Kavurmaci O, Ergonul AG, et al. Analysis of the patients with simultaneous bilateral spontaneous pneumothorax. Clin Respir J 2018;12:1207-11. [Crossref] [PubMed]
- Cho DG, Lee SI, Chang YJ, et al. Thoracoscopic Bilateral Bullectomy for Simultaneously Developed Bilateral Primary Spontaneous Pneumothorax: Ipsilateral Transmediastinal versus Bilateral Sequential Approach. Thorac Cardiovasc Surg 2017;65:56-60. [PubMed]
- Chen YJ, Luh SP, Hsu KY, et al. Video-assisted thoracoscopic surgery (VATS) for bilateral primary spontaneous pneumothorax. J Zhejiang Univ Sci B 2008;9:335-40. [Crossref] [PubMed]
- Luh SP. Diagnosis and treatment of primary spontaneous pneumothorax. J Zhejiang Univ Sci B 2010;11:735-44. [Crossref] [PubMed]
- Donahue DM, Wright CD, Viale G, et al. Resection of pulmonary blebs and pleurodesis for spontaneous pneumothorax. Chest 1993;104:1767-9. [Crossref] [PubMed]
- Hazelrigg SR, Landreneau RJ, Mack M, et al. Thoracoscopic stapled resection for spontaneous pneumothorax. J Thorac Cardiovasc Surg 1993;105:389-92. [PubMed]
- Noppen M, De Keukeleire T. Pneumothorax. Respiration 2008;76.121-7.
- Huang TW, Cheng YL, Tzao C, et al. Factors related to primary bilateral spontaneous pneumothorax. Thorac Cardiovasc Surg 2007;55:310-2. [Crossref] [PubMed]
- Yamada S, Yoshino K, Inoue H. Simultaneous bilateral spontaneous pneumothorax with pleural window communicating with bilateral pleural spaces. Ann Thorac Surg 2008;85:1434-6. [Crossref] [PubMed]
- Hata Y, Suzuki T, Yokoi M, et al. Simultaneous bilateral spontaneous pneumothorax with congenital pleuro-pleural communication. J Thorac Dis 2013;5:87-9. [PubMed]
- Casali C, Stefani A, Ligabue G, et al. Role of blebs and bullae detected by high-resolution computed tomography and recurrent spontaneous pneumothorax. Ann Thorac Surg 2013;95:249-55. [Crossref] [PubMed]
- Ouanes-Besbes L, Golli M, Knani J, et al. Prediction of recurrent spontaneous pneumothorax: CT scan findings versus management features. Respir Med 2007;101:230-6. [Crossref] [PubMed]
- Sihoe AD, Yim AP, Lee TW, et al. Can CT scanning be used to select patients with unilateral primary spontaneous pneumothorax for bilateral surgery? Chest 2000;118:380-3. [Crossref] [PubMed]
- Martínez-Ramos D, Angel-Yepes V, Escrig-Sos J, et al. Usefulness of computed tomography in determining risk of recurrence after a first episode of primary spontaneous pneumothorax: therapeutic implications. Arch Bronconeumol 2007;43:304-8. [Crossref] [PubMed]
- Huang TW, Lee SC, Cheng YL, et al. Contralateral recurrence of primary spontaneous pneumothorax. Chest 2007;132:1146-50. [Crossref] [PubMed]
- Chou SH, Li HP, Lee JY, et al. Is prophylactic treatment of contralateral blebs in patients with primary spontaneous pneumothorax indicated? J Thorac Cardiovasc Surg 2010;139:1241-5. [Crossref] [PubMed]
- Chen YY, Huang HK, Chang H, et al. Postoperative predictors of ipsilateral and contralateral recurrence in patients with primary spontaneous pneumothorax. J Thorac Dis 2016;8:3217-24. [Crossref] [PubMed]
- Graf-Deuel E, Knoblauch A. Simultaneous bilateral spontaneous pneumothorax. Chest 1994;105:1142-6. [Crossref] [PubMed]
- Ayed AK. Bilateral video-assisted thoracoscopic surgery for bilateral spontaneous pneumothorax. Chest 2002;122:2234-7. [Crossref] [PubMed]
- Song N, Jiang G, Xie D, et al. Bilateral bullectomy through uniportal video-assisted thoracoscopic surgery combined with contralateral access to the anterior mediastinum. J Bras Pneumol 2013;39:32-8. [Crossref] [PubMed]
- Kim KS. Single-staged uniportal VATS in the supine position for simultaneous bilateralprimary spontaneous pneumothorax. J Cardiothorac Surg 2017;12:25. [Crossref] [PubMed]
- Flores-Franco RA, Llore-Gonzalez A. Treatment of bilateral spontaneous pneumothorax: the catheter drainage method is still useful! Respirology 2008;13:1093-4. [PubMed]
- Ikeda M, Uno A, Yamane Y, et al. Median sternotomy with bilateral bullous resection for unilateral spontaneous pneumothorax, with special reference to operative indications. J Thorac Cardiovasc Surg 1988;96:615-20. [PubMed]
- Wang X, Wang L, Wang H, et al. Simultaneous Bilateral Video-Assisted Thoracoscopic Surgery for the Treatment of Primary Spontaneous Pneumothorax. Cell Biochem Biophys 2015;71:1703-8. [Crossref] [PubMed]
- Guo Z, Yin W, Zhang X, et al. Primary spontaneous pneumothorax: simultaneous treatment by bilateral non-intubated videothoracoscopy. Interact Cardiovasc Thorac Surg 2016;23:196-201. [Crossref] [PubMed]
- Yang HC, Cho S, Jheon S. Single-incision thoracoscopic surgery for primary spontaneous pneumothorax using the SILS port compared with conventional three-port surgery. Surg Endosc 2013;27:139-45. [Crossref] [PubMed]
- Liu C, Ma L, Lin F, et al. Single-staged uniportal VATS major pulmonary resection for bilateral synchronous multiple primary lung cancers. J Thorac Dis 2014;6:1315-8. [PubMed]
- Wu YC, Chu Y, Liu YH, et al. Thoracoscopic ipsilateral approach to contralateral bullous lesion in patients with bilateral spontaneous pneumothorax. Ann Thorac Surg 2003;76:1665-7. [Crossref] [PubMed]
- Liu CC, Wang BY, Shih CS, et al. Subxiphoid single-incision thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg 2014;148:3250-1. [Crossref] [PubMed]
- Hernandez-Arenas LA, Lin L, Yang Y, et al. Initial experience in uniportal subxiphoid video-assisted thoracoscopic surgery for major lung resections. Eur J Cardiothorac Surg 2016;50:1060-6. [Crossref] [PubMed]
- Chen L, Liu F, Wang B, et al. Subxiphoid vs transthoracic approach thoracoscopic surgery for spontaneous pneumothorax: a propensity score-matched analysis. BMC Surg 2019;19:46. [Crossref] [PubMed]
- Li L, Tian H, Yue W, et al. Subxiphoid vs intercostal single-incision video-assisted thoracoscopic surgery for spontaneous pneumothorax: A randomised controlled trial. Int J Surg 2016;30:99-103. [Crossref] [PubMed]
Cite this article as: Liu YW, Chou SH. Surgery for simultaneous bilateral primary spontaneous pneumothorax: when and how? Video-assist Thorac Surg 2019;4:21.


