
Should we screen patients undergoing thoracic surgery for aortic stenosis pre-operatively?
Introduction
Aortic stenosis (AS) is characterized as a high-risk indicator for cardiac complications during non-cardiac surgeries. Whilst at present factors such as arrhythmias, heart failure, recent myocardial infarction and ischaemic heart disease are well-established identifiable risk factors for these peri-operative complications, AS is, at present, not included in cardiac risk prediction models due to its under diagnosis and under-representation in databases and resulting risk assessment tools (1). Despite this, severe AS is the most prevalent valvular heart disease in the elderly; such cohort often requires non-cardiac surgery for other pathologies, such as lung cancer.
AS results in an obstruction in the outflow tract that overtime leads to left ventricular myocardial hypertrophy (1). At first, the cardiac output and left ventricular end-diastolic volumes are preserved, meaning that patients are asymptomatic. However, overtime, the concentric left ventricular hypertrophy and reduces compliance of the myocardium which leads to diastolic dysfunction. This results in symptoms such as chest pain or dyspnea as a result of the increased diastolic pressure of the left ventricle (1). Moreover, AS causes systemic hypotension and a reduction in coronary flow reserve which can counts for the angina type chest pain (1). Thoracic surgery serves as a mechanism by which haemodynamic stress is further imposed on these patients, serving as a risk of resultant decompensated heart failure.
Current guidelines set out by the European Society of Cardiology (ESC), the European Association of Cardio-Thoracic Surgery (EACTS), and the American College of Cardiology (ACC)/American Heart Association (AHA) which outlines the decision-making process in patients undergoing non-cardiac surgery for patients with severe AS. Asymptomatic patients that are with low/moderate surgical risks are considered to be fit to proceed with non-cardiac surgery (2-4). However, these guidelines also recommend that in patients who have severe symptomatic AS, cancelling or postponing the non-cardiac surgery should be considered (2-4). For symptomatic patients, the AHA guidelines suggest postponing non-cardiac operations if the valve has not been evaluated within a year. While these guidelines are useful, one should consider them with caution as they are largely based on small and not very recent observational studies.
Postponing or cancelling required surgery has major implications on both patient’s prognosis from non-cardiac disease which may need operative intervention and on patient’s perception of their illness and the means to overcome it (1). Screening for AS, as a result, has a role in detecting asymptomatic patients prior to their development of severe symptomatic illness (1). As the majority of the population will require any operation at any given time, this may prevent the need to cancel or postpone operations that could have otherwise taken place if intervention for AS may have occurred earlier.
Screening for AS in patients undergoing thoracic surgery
AS follows a lengthy course over years during which patients are often unaware of their condition and mortality rates increase dramatically soon after the onset of symptoms (1). As a result, both patient and physician awareness need to be increased and methods of early diagnosis rates and referral need to be improved, to early recognition of the pathology and treat it promptly. AS commonly presents firstly in the primary care setting, and as a result, family physicians or general practitioners play a key role in the timely diagnosis and referral of patients with suspected AS.
The disease burden of AS is increasing with the current aging population (5). A diagnosis of severe symptomatic AS is associated with an average life expectancy of 2–3 years and therefore it requires a timely valve intervention. Whilst symptomatic patients are more likely to receive timely intervention, asymptomatic patients are less likely to acquire the needed interventions (6).
In patients with an established diagnosis of AS however, only two-thirds of those meeting guideline recommendations for valve replacement therapy actually receive treatment, with failure to intervene usually due to an overestimation of the risks involved, underestimation of symptoms or misclassification of the severity of the AS (7). Operatively, managing the AS may differ between receiving a thoracic intervention on time or delaying treatment due to increased surgical risk conferred by the AS (8). Screening plays a role in these individuals with AS who may otherwise present late; either due to being asymptomatic, whose symptoms are being underestimated or those who are not being followed-up post diagnosis regarding their disease severity and progression.
Recent data shows that patients are only diagnosed when they develop symptoms, as this precipitates the referral for echocardiography (7). Valve replacement therapy however may have been suitable for these patients prior to the onset of their symptoms. Current guidelines recommend that valve replacement surgery is indicated in those with impaired left ventricular ejection fraction (LVEF <50%) or in those whom exercise testing unmasks symptoms but also in those with very severe AS (Vmax >5.5 m/s), severe valve calcification and evidence of fast progression, pulmonary hypertension or markedly elevated brain natriuretic peptide (BNP) levels (9). These indications may be otherwise missed, highlighting the importance of introducing a screening scheme.
There is accumulating literature which reports the benefit of early intervention in patients with asymptomatic severe AS, as symptom onset may signify a critical timepoint in the course of the disease.
Based on echocardiographic findings such as transvalvular gradient, flow velocity and effective orifice area, the degree of AS usually progresses as an asymptomatic condition from mild, moderate to severe (7).
The difficulty with waiting to treat AS at the point where patients become symptomatic is that these symptoms are nonspecific for AS alone and these symptoms can result from other conditions; as a result, the threshold for screening and referral should be low (7).
Screening for AS can be performed through different modalities, starting from initial clinical assessment to imaging studies. The initial screening through patient symptoms can guide further assessment; patients with AS present with angina, shortness of breath, dizziness or lightheadedness and loss of consciousness. Some patients remain asymptomatic and are found to have AS incidentally. However, the gold standard method for diagnosis of AS is through echocardiography where it can assess the severity of the pathology and measure the pressure gradient (7).
Operative management of lung cancer and AS
Traditionally, in patients with resectable lung malignancies, it has been recommended that AS is treated prior to the lung resection (2-4,10). With this approach however, the tumour resection is delayed and there is consequently an increase in morbidity rates.
If surgical treatment of both lung cancer and AS confers an increased surgical risk, surgical management of AS has been reported to be prioritized over lung resection (11). However, the presence of severe AS in surgical patients, including those undergoing thoracic surgery, translates to a 5% risk of major adverse cardiovascular events occurring. It has been suggested that by undergoing preceding transcatheter aortic valve implantation (TAVI), patients may safely undergo lung resection as well as manage their AS (11). Sakai et al. (11) presented cases whereby high-risk patients were treated for both severe AS and lung cancer by performing TAVI and lung resection. These cases are summarised in Table 1.
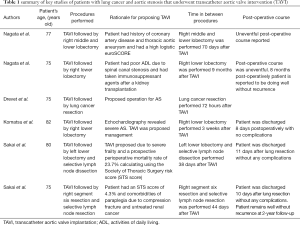
Full table
A further study by Nagata et al. (12) outlines two cases where patients underwent lobectomy for lung cancer after TAVI. These cases are outlined in Table 1. A further case report by Drevet et al. (13) outlines the management of a 75-year-old patient with AS and lung cancer who underwent left upper sleeve lobectomy 72 hours after TAVI. Post operatively, this patient had complications of pneumonia and atrial fibrillation, however a year after lobectomy, the patient was free from recurrence of the tumour.
All these case reports document successful management of AS and lung cancer, with no recurrence and it can put a ground of successful management of both pathologies safely with optimum outcome.
Current literature supports the use of TAVI in patients undergoing cancer surgery as it does not require cardiopulmonary bypass (CPB). This is because the use of CPB for cancer patients can confer a risk of progression of cancer through the dissemination of cancer cells (14). Based on this advantage, as well as reduced time intervals enabled by TAVI, pulmonary resection followed by TAVI can be safely performed and this strategy is a viable option for patients with both lung cancer and severe AS, for whom conventional AVR by open-heart surgery is not indicated or high risk (12-14).
Performing cardiac valve replacement and pulmonary resection simultaneously may be considered advantageous, as an alternative, as it avoids a secondary procedure, yet this can add incremental risk factors and issues with accessing the appropriate site of the lesion (15).
In patients with lung cancer amenable to surgery, resection offers the best hope for cure. In regard to concomitant pulmonary surgical resection, left lower lobectomy is technically the most challenging resection to perform as the dissection is obscured by the heart and retraction can induce dysrhythmias and haemodynamic instability. This resection can be facilitated by extending the incision laterally into the intercostal space (16).
Furthermore, access to the posterior mediastinum for lymph node sampling is possible although more difficult when compared with that of thoracotomy. It is recommended that a separate posterolateral thoracotomy may still be required for certain tumours invading the chest wall. However, the creation of two incisions confers an increased mortality rate and post-operative pain (16).
Patients who undergo heart surgery with CPB are at substantial risk of postoperative bleeding. Bleeding can result from excessive heparinization, inadequate heparin neutralization or low protamine dose. More commonly it is due to a transient impairment of platelet function due to platelet activation during the passage through the extra-corporeal circuit. In patients undergoing a concomitant procedure, the bleeding may arise from both the area of the lung resection and the mediastinal node dissection and accounts for a significant cause of postoperative morbidity (16). Means of reducing this risk includes performing anatomical resections of the lung over wedge excisions, performing the procedure without CPB where possible, and performing the lung resection prior to heparinization and its institution.
Pulmonary dysfunction sustained during CPB may adversely affect the outcome in patients undergoing simultaneous lung resection and cardiac surgery. Fluid overload, inflammation and endothelial cell injury all contribute to this resultant lung injury (16). In patients with coexistent lung disease such as lung cancer, these effects may become more important.
By avoiding CBP in these patients, the lung injury is amended and early extubation is possible. Other means of reducing this effect on the lungs includes reducing fluid retention by haemoconcentration and leucocyte infiltration (16).
There has been concern regarding the effect of CPB on malignant growth and its effects on the long-term survival of patients with coexisting cancer. Several authors have demonstrated that in patients undergoing combined surgery, long-term survival is prolonged in patients whose lung cancer is resected prior to open heart surgery compared with simultaneous resection and open-heart surgery. However, despite this, there isn’t an increased risk of cancer recurrence in patients who have been previously treated for a malignancy and then undergo cardiac surgery (16).
Should we intervene prior to operation in symptomatic patients undergoing thoracic surgery?
Patients with severe AS who require non-cardiac surgery present a difficult clinical problem. Their rate of postoperative cardiovascular complications is increased in comparison to patients without AS (5). The most appropriate management still remains uncertain. In elderly patients, who are more prone to develop symptomatic AS, careful pre-operative assessment is necessary to determine the severity of the AS, as well as stratification of the symptoms that are attributable to AS and the related risks/benefits associated with the proposed operation (5). The main challenge at present is to identify which patients would be suitable for aortic valve intervention prior to non-cardiac surgery.
No trials have been published at present that have investigated whether or not the overall risk of aortic valve intervention followed by non-cardiac surgery is lower than the risk of undertaking non-cardiac surgery alone without prior valve intervention (5). This, as a result, has led for the published guidelines to base themselves on consensus of opinions (5).
A study by Agarwal et al. (8) assessed to determine the impact of AS on the post-operative outcomes of patients after non-cardiac surgery. The study matched 634 patients with AS undergoing non-cardiac surgery to 2,536 controls. There were 244 patients with severe AS and 390 with moderate AS. The study showed that the 30-day mortality in patients with AS was 2.1% in comparison to 1.0% in non-AS controls (P=0.036). Postoperative myocardial infarction was more frequent in patients with AS compared with controls (3.0% vs. 1.1%; P=0.001). Combined primary outcome was significantly worse for both moderate and severe AS patients compared with respective controls (4.4% vs. 1.7%; P=0.002; and 5.7% vs. 2.7%; P=0.02, respectively). The study concluded that the presence of AS is detrimental regarding postoperative outcomes among patients undergoing non-cardiac surgery, evidenced by a higher 30-day mortality and postoperative myocardial infarction after non-cardiac surgery.
Furthermore, a study by Kertai et al. (17) demonstrated that the severity of AS was highly predictive of perioperative mortality and non-fatal myocardial infarction. The outcome measure was the composite of perioperative mortality and nonfatal myocardial infarction. The study of 324 patients (108 with moderate-severe AS and 216 controls) showed that there was a significantly higher incidence of the composite endpoint in patients with AS than in patients without AS [14% (15/108) vs. 2% (4/216) respectively, P<0.001]. The rate of perioperative complications was substantially higher in patients with severe AS compared to patients with moderate AS [31% (5/16) vs. 11% (10/92), P=0.04].
Both the ACC/AHA and the ESC guidelines highlight the importance of general measures in the management of patients with untreated valvular heart disease who are undergoing non-cardiac surgery (2-5). They recommend the careful selection of mode of anaesthesia, the use of invasive haemodynamic monitoring, avoidance of rapid changes in volume status, treating any arrhythmias and high intensity postoperative care (2-5).
Regarding preoperative valve intervention, the European guidelines enforce preoperative valve intervention. For patients with symptomatic severe AS, aortic valve replacement (AVR) is recommended prior to elective non-cardiac surgery, provided that the valve surgery would not involve a high risk. In patients with symptomatic severe AS for whom AVR would involve a high risk, balloon aortic valvuloplasty (BAV), or TAVI is recommended (5,18,19). They recommend that the choice between TAVI and BAV is made based on the anticipated life expectancy and urgency of the proposed non-cardiac surgery. The main evidence for TAVI prior to the publication of current clinical practice guidelines for the perioperative management of patients undergoing non-cardiac surgery came from the first PARTNER trial (20); this showed that TAVI is as effective as AVR in patients with severe AS who are at high-risk of complications from AVR. At present, TAVI has numerous advantages over AVR: the procedural mortality rate is lower in high- and intermediate-risk patients, it is less invasive, and the recovery time is quicker which facilitates early non-cardiac surgery after the procedure (5).
For asymptomatic patients who have severe AS, the recommendations vary according to the risk of the proposed non-cardiac surgery. Patients who are undergoing high-risk non-cardiac operations are recommended to undergo AVR prior to these taking place (2-5).
Based on these recommendations, the only thoracic procedure which at present carries a greater risk is a pneumonectomy, with a 30-day mortality rate of 5% to 10% (21). Most of the remaining thoracic procedures are considered intermediate- or low-risk, based on cardiac risk stratification (1% to 5%). For these patients, no aortic valve intervention is recommended if the risks of the operation are deemed to be acceptable (2-5).
Current recommendations emphasize the need to implement clinical judgement when assessing which patients require intervention. Elective surgery should only be performed if it is strictly necessary and using invasive haemodynamic monitoring.
Conclusions
Screening for AS prior to thoracic surgery plays a key role in optimizing perioperative outcomes in patients with lung cancer. In patients undergoing thoracic surgery, a transthoracic echocardiograph suffices as a screening tool for AS. For those patients who are found to have severe AS at screening stage, TAVI is an appropriate intervention prior to thoracic surgery with low complication rates. Clinical judgment should be implemented when assessing which patients require surgical intervention.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2019.06.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Osnabrugge RL, Kappetein AP, Serruys PW. Non-cardiac surgery in patients with severe aortic stenosis: time to revise the guidelines? Eur Heart J 2014;35:2346-8. [Crossref] [PubMed]
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery. Circulation 2007;116:e418-99. [PubMed]
- Poldermans D, Baxx JJ, Boersma E, et al. Guidelines for Pre-Operative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-Cardiac Surgery: the Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery of the European Society of Cardiology (ESC) and endorsed by the European Society of Anaesthesiology (ESA). Eur J Anaesthesiol 2010;27:92-137. [Crossref] [PubMed]
- Kappetein AP, Torbicki A, Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2012;33:2451-96. [Crossref]
- Kennon S, Archbold A. Guidelines for The Management of Patients with Aortic Stenosis Undergoing Noncardiac Surgery: Out of Date and Overly Prescriptive. Interv Cardiol 2017;12:133-6. [PubMed]
- Christophe T, Dan R, Yohann B, et al. How Should Very Severe Aortic Stenosis Be Defined in Asymptomatic Individuals? J Am Heart Assoc 2019;8:e011724 [PubMed]
- Thoenes M, Bramlage P, Zamorano P, et al. Patient screening for early detection of aortic stenosis (AS)-review of current practice and future perspectives. J Thorac Dis 2018;10:5584-94. [Crossref] [PubMed]
- Agarwal S, Rajamanickam A, Bajaj NS, et al. Impact of Aortic Stenosis on Postoperative Outcomes After Noncardiac Surgeries. Circ Cardiovasc Qual Outcomes 2013;6:193-200. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- British Thoracic Society, Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 2001;56:89-108. [Crossref] [PubMed]
- Sakai T, Yahagi K, Miura S, et al. Transcatheter aortic valve implantation for patients with lung cancer and aortic valve stenosis. J Thorac Dis 2018;10:E387-90. [Crossref] [PubMed]
- Nagata H, Kanzaki R, Kanou T, et al. Two cases of lobectomy for lung cancer after transcatheter aortic valve implantation. Surg Case Rep 2018;4:139. [Crossref] [PubMed]
- Drevet G, Maury JM, Farhat F, et al. Transcatheter Aortic Valve Implantation: A Safe and Efficient Procedure to Treat an Aortic Valve Stenosis Before Lung Cancer Resection. Gen Thorac Cardiovasc Surg 2019;67:321-3. [Crossref] [PubMed]
- Komatsu H, Izumi N, Tsukioka T, et al. Pulmonary Resection for Lung Cancer Following Transcatheter Aortic Valve Implantation for Severe Aortic Valve Stenosis: A Case Report. Ann Thorac Cardiovasc Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Rao V, Todd TR, Weisel RD, et al. Results of Combined Pulmonary Resection and Cardiac Operation. Ann Thorac Surg 1996;62:342-6. [Crossref] [PubMed]
- Danton MH, Anikin VA, McManus KG, et al. Simultaneous cardiac surgery with pulmonary resection: presentation of series and review of literature. Eur J Cardiothorac Surg 1998;13:667-72. [Crossref] [PubMed]
- Kertai MD, Bountioukos M, Boersma E, et al. Aortic stenosis: an underestimated risk factor for perioperative complications in patients undergoing noncardiac surgery. Am J Med 2004;116:8-13. [Crossref] [PubMed]
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014;35:2383-431. [Crossref] [PubMed]
- Svensson LG, Tuzcu M, Kapadia S, et al. A comprehensive review of the PARTNER trial. J Thorac Cardiovasc Surg 2013;145:S11-6. [Crossref] [PubMed]
- Powell ES, Pearce AC, Cook D, et al. UK pneumonectomy outcome study (UKPOS): a prospective observational study of pneumonectomy outcome. J Cardiothorac Surg 2009;4:41. [Crossref] [PubMed]
- Fuentes PA. Pneumonectomy: historical perspective and prospective insight. Eur J Cardiothorac Surg 2003;23:439-45. [Crossref] [PubMed]
Cite this article as: Bithas C, Harky A. Should we screen patients undergoing thoracic surgery for aortic stenosis pre-operatively? Video-assist Thorac Surg 2019;4:15.


