
Trans-subxiphoid robot-assisted thoracoscopic surgery for resecting multiple thymomas: a case report
Introduction
Multiple thymoma (MT) is known to be a quite rare disease (1-3). The genesis of MTs may involve both intrathymic metastasis and multicentric tumors. Most of MTs have the same histotype, and the relatively small number of MTs having various histotypes are also found (1-5).
Minimally invasive thymectomy by video-assisted thoracoscopic surgery (VATS) is performed for resecting thymoma. However, the lack of perspective sensation by two-dimensional view and the restricted movements of surgical instruments give difficulty in handling the tissues. Robotic-assisted thoracoscopic surgery (RATS) thymectomy have more technical advantages such as a three-dimensional (3D) vision system and multi-articulated instruments (6). Trans-subxiphoid RATS thymectomy can provide a better surgical view in the upper poles of thymus and bilateral phrenic nerves (7).
Here in, a clinical case where MT with different histotypes were resected by trans-subxiphoid RATS thymectomy was reported.
Case presentation
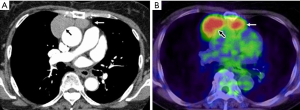
Patient was a 67-year-old woman who was found to have an abnormal shadow by chest radiograph during her medical checkup. Computer tomography (CT) found two masses in the anterior mediastinum, and the patient was referred to the authors’ hospital. No symptoms including neurological manifestations were found, no abnormal findings were found in laboratory data, and anti-acetylcholine antibody was negative. Chest CT found two circular-like shape masses with sizes of 47 mm × 43 mm and 31 mm × 18 mm, of which surfaces and interior statuses were smooth and homogenous, in the anterior mediastinum (Figure 1A), and no contrast effects were observed in the tumors. 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) found two sharply marginated and regularly bordered masses in the anterior mediastinum (Figure 1B), the right and left side lesions showed fluorodeoxyglucose (FDG) accumulations with a maximum standardized uptake values (SUVmax) of 4.80 and 4.21, respectively. No significant swelling of lymph nodes and no abnormal FDG accumulation were observed in the lymph nodes.
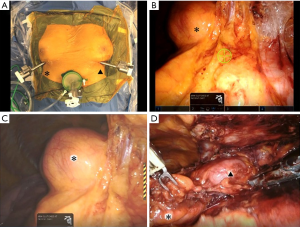
The patient was laid in a supine position under general anesthesia and one-lung ventilation, a 4-cm skin incision is made above the subxiphoid, and GelPOINT Mini (Applied Medical, Rancho Santa Margarita, CA, USA) was affixed for a camera scope. Camera scope was inserted with CO2 gas injection at a pressure of 6 mmHg by an AirSeal® intelligent flow system (ConMed, Milford, CT). Two 8-mm ports for the da Vinci robotic arm were inserted bilaterally into a 1-cm skin incision in the sixth intercostal space on the mid-claviculate line (Figure 2A). The robotic surgical system (da Vinci Xi®) (Intuitive Surgical, Sunnyvale, CA) was docked over the left side head of the patient. The da Vinci camera scope was mounted through the subxiphoid port (Figure 2B). A monopolar spatula was mounted on the right arm, and a pair of bipolar fenestrated grasping forceps was mounted on the left arm. Appropriately, the spatula was replaced with a bipolar vessel sealing system. Right lobe of thymus including the right side thymoma was separated from the right phrenic nerve and pericardium (Figure 2C), and the left lobe of thymus was also separated in the same manner (Figure 2D). Two thymomas were resected as a mass with the thymus, which were placed in a bag in the mediastinum and removed through the subxiphoid incision. Silicon tubes with an inner and outer diameter of 4.2 and 6.7 mm, respectively, (Thoracic Catheter, Straight) (REDAX, Poggio Rusco, Italy) were inserted through both the sixth intercostal space ports.
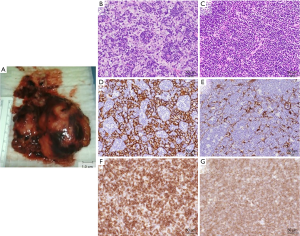
The resected thymomas were found to be oval or ellipse in shape with sizes of 5 cm × 4 cm × 2.5 cm and 5 cm × 3 cm × 1.5 cm (Figure 3A), and cells having clearly distinguishable nuclei proliferated with small-size lymphocytes in the tumors (Figure 3B,C). Immunohistochemical examination showed that the tumors were positive for CK (AE1/3), CD3, and CD1a (Figure 3D,E,F,G). The larger tumor was diagnosed to be type B1 thymoma, because it was rich in lymphocytes, and the smaller tumor was type B2 thymoma because of the proliferation of epithelial cells. Both thymomas were in the stage II of the Masaoka staging system.
Discussion
MT is reported to be a quite rare disease, which occupies only 1.1–2.2% of whole thymomas (1-3). From 1961, 26 MT cases are reported, and 23 cases have precise record containing thymomas’ histotypes and their disease stages. Among them, 19 cases (82.6%) resect two thymomas; 3 cases (13.0%), three thymomas; 1 case (4.3%), six thymomas (5). In the total 53 resected thymomas in 23 cases, the number of type A thymomas is 5 (9.4%); type AB, 12 (22.6%); type B1, 21 (39.6%); type B2, 12 (22.6%); type B3, 1 (1.9%); type B2+3, 1 (1.9%); micronodular thymoma with lymphoid stroma (MNT), 1 (1.9%). In the Masaoka staging system, the number of thymoma at the stage I is 38 (71.7%); the stage II, 13 (24.5%); the stage III, 2 (3.8%).
MT with myasthenia gravis is also reported, and the complication rate is found to be 30.4–37.5% of total number of MT (4,8). Histologically, 65.2–83.0% resected thymomas show the same histotype (2,4,5). As the origin of MT, multicentric development and intrathymic metastasis types are considered. The resected thymomas having different histotypes are diagnosed to be multicentric development type thymomas. However, if the thymoma has the same histotype, it is difficult to diagnose which the origin of tumor is, multicentric development origin or intra-thymic metastasis origin. In previous report, since the nucleus morphology in thymus epithelium and the effect of immuno-staining on the tumor is identical each other, thymomas are diagnosed as an intrathymic metastasis origin thymoma (9). As the characteristics of multicentric development origin thymomas, the number of thymomas is two, the sizes of the thymomas resemble each other, and the most resected thymomas are diagnosed to be the stage I of the Masaoka stage (10).
As a treatment, thymomas are removed surgically if it resectable. Although thymectomy is conventionally performed by median sternotomy, recently, as a minimally invasive surgical approach, VATS is performed. Compared with conventional median sternotomy, VATS thymectomy is reported to reduce the incidence of postoperative complications, shorten the length of postoperative hospital stay, and give a lower incidence of lung functional deterioration that is frequently observed after conventional median sternotomy (11,12). However, VATS thymectomy is reported to give a slightly higher incident rate of phrenic nerve damages (6.7%) than that of the conventional median sternotomy (0%) due to poor visual fields on mediastinal fat and phrenic nerves (13). Suda et al. reported that subxiphoid approaching VATS gives a better visual field on the superior pole of thymus and phrenic nerves (7).
The robotic surgical system, which can give more flexibility to operating arms with multi-joint forceps, allows surgeons to perform their operating maneuvers easily (6), and RATS for thymomas is reported to be safe and easily performed (12,14). On the other hand, unilateral approaching RATS is mentioned to be difficult to confirm the existence of the brachiocephalic vein in the opposite side of chest. Trans-subxiphoid RATS thymectomy gives a good visual field on the left brachiocephalic vein, and even in a case where the invasion of tumors is suspected, the robotic surgical system allows surgeons to tape the left brachiocephalic vein.
Moreover, in a case where the invasion is found to reach to the pericardium, the pericardial resection and the reconstruction of pericardium are difficult to be performed by VATS, which is unable to be applied thymectomy. However, RATS, which can maneuver multi-articulated forceps, can perform minimally invasive operation for thymectomy (15).
In this report, trans-subxiphoid RATS thymectomy was performed for a MT found in the thymic gland. This procedure was confirmed to be applicable for both thoracic cavities and performed quickly and easily with a wide visual field, resulting in the achievement of more minimally invasive surgery.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2019.06.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bernatz PE, Harrison EG, Clagett OT. Thymoma: a clinicopathologic study. J Thorac Cardiovasc Surg 1961;42:424-44. [PubMed]
- Mori T, Nomori H, Ikeda K, et al. Three cases of multiple thymoma with a review of the literature. Jpn J Clin Oncol 2007;37:146-9. [Crossref] [PubMed]
- Kawaguchi K, Usami N, Uchiyama M, et al. Triple thymoma with different histologic types. J Thorac Cardiovasc Surg 2007;133:826-7. [Crossref] [PubMed]
- Yokota K, Kanzaki M, Miyano Y, et al. A case of myasthenia gravis with multiple thymoma. Respiratory Research 2008;27:180-1. (In Japanese).
- Inafuku K, Nishii T, Ito H, et al. A surgical case of synchronous multiple thymoma of multicentric origin. Jpn J Chest Surg 2018;32:78-83. [Crossref]
- Kanzaki M. Current status of robot-assisted thoracoscopic surgery for lung cancer. Surg Today 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Suda T, Tochii D, Tochii S, et al. Trans-subxiphoid robotic thymectomy. Interact Cardiovasc Thorac Surg 2015;20:669-71. [Crossref] [PubMed]
- Seo DH, Cho S. Multiple thymoma with myasthenia gravis. Korean J Thorac Cardiovasc Surg 2017;50:68-70. [Crossref] [PubMed]
- Nomori H, Kobayashi K, Ishihara T, et al. A case of multiple thymomas: the possibility of intra-thymic metastasis. Jpn J Clin Oncol 1990;20:209-11. [Crossref] [PubMed]
- Suzuki H, Yoshida S, Hiroshima K, et al. Synchronous multiple thymoma: report of three cases. Surg Today 2010;40:456-9. [Crossref] [PubMed]
- Chetty GK, Khan OA, Onyeaka CVP, et al. Experience with video-assisted surgery for suspected mediastinal tumors. Eur J Surg Oncol 2004;30:776-80. [Crossref] [PubMed]
- Maeda H, Isaka T, Mitsuboshi S, et al. Minimally invasive surgery for thymic epithelial tumors: a single institutional experience. Video-assist Thorac Surg 2017;2:47. [Crossref]
- Xie A, Tjahjono R, Phan K, et al. Video-assisted thoracoscopic surgery versus open thymectomy for thymoma: a systematic review. Ann Cardiothorac Surg 2015;4:495-508. [PubMed]
- Casiraghi M, Galetta D, Borri A, et al. Robotic-assisted thymectomy for early stage thymoma: a propensity-score matched analysis. J Robot Surg 2018;12:719-24. [Crossref] [PubMed]
- Suda T. Robotic subxiphoid thymectomy. J Vis Surg 2016;2:118. [Crossref] [PubMed]
Cite this article as: Aoshima H, Isaka T, Yamamoto T, Kanzaki M. Trans-subxiphoid robot-assisted thoracoscopic surgery for resecting multiple thymomas: a case report. Video-assist Thorac Surg 2019;4:14.


