Simulation for the video-assisted thoracic surgery surgeon
Introduction
Video-assisted thoracic surgery (VATS) has emerged since its beginning in the 1990s to a point where most of procedures can be performed by this minimally invasive technique.
There is substantial evidence that VATS offer advantages compared to thoracotomy such as patient’s safety, shorter length of hospitalization, better outcome, decreased trauma, and reduced post-operative morbidity (1,2). VATS surgeons must have extensive knowledge and skills to operate on patients. Learning VATS to a competency level where the next generation of thoracic surgeons can operate patients is a challenging task. It is suggested that trainees perform 100 minor procedures to get familiar with the surgical instruments and basic VATS skills (3). There are different opinions about the amount of procedures to reach the required level of competency and in fact it is impossible to determine a precise number of operations necessary to become a competent VATS surgeon. A threshold of 50 procedures will not ensure that all surgeons are competent. All learning curves are different and surgeons who start from scratch have more challenges than surgeons who learn VATS surgery supervised by an experienced VATS surgeon. The size of the center and the potential number of operations also influence the learning curve as performing more operations in a short interval will shorten the learning curve (3,4). Different types of simulators have been developed to facilitate more rapid learning in a simulated, risk free and time-efficient manner. The aim of the current review was to get a comprehensive overview of the different simulation modalities and to explore the existing evidence of their training efficacy.
Materials and methods
Electronic searches were performed in PubMed and Google scholar from their inception to December 2018. To achieve the maximum sensitivity of search strategy and identify all trials of simulation, we used the following terms VATS and simulation, Simulation training thoracic, VATS and thoracoscopy simulation, Simulation and thoracoscopy, VATS lobectomy simulation. All the studies including VATS simulation published in English were included. The reference of all retrieved articles was reviewed for further identification of any relevant studies.
We excluded studies that were directly not relevant for this study. No restrictions were placed on abstract proceeding. A total of 454 articles and abstracts were found. One hundred and seventeen articles were duplicates. After reviewing all these articles, 33 articles were eligible for our study (Figure 1).
Results
The included studies identified three different simulation modalities: dry lab simulators, wet lab simulators, and virtual reality simulators.
Dry lab simulators
Dry-lab simulators are relatively inexpensive and commonly used among trainees. Normal surgical instruments may be used, and haptic feedback is preserved which contributes to the reality of training (5). Box trainers increase the skills and enable novices to learn and perform basic procedures (5,6). There are many box trainers currently available. The majority is used to acquire the basic VATS skills, but there are new simulators including disposable artificial lungs or human rib cage model with bony ribs and polyvinyl-alcohol hydrogel (PVA) lungs (7). These new models can be expensive but create high fidelity for VATS training.
Wet lab simulators
Wet lab simulators are very sophisticated and create a realistic setting, but have restricted availability due to its costs, preparation and ethical concerns (5). Swine are commonly used for simulation but apart from the expenses and ethical issues they also have poor cardiopulmonary reserve, which make anesthesia difficult and their anatomy differs a lot from human’s anatomy. Tedde et al. used 40 swine in an advanced course, where they had challenges with anesthesia, single lung ventilation and anatomical differences.
Sheep anatomy is closer to the human. The right lung is a little different, the caval vein is bigger and the arterial branches are behind it and the upper lobe has a tracheal bronchus. The left lung is small with a long lingula, but many believe it is more similar to human than the right lung (8,9). Animal models are expensive and time consuming for preparation.
Virtual reality simulators
Virtual reality (VR) simulators create realistic settings and can teach the technical aspect of procedure in an environment where the trainees can achieve surgical competence before performing it on a patient (9). VR simulations allow the novice surgeon to develop their surgical skills such as hand-eye coordination, depth perception, movement of instruments, interaction of dominant hand, psychomotor skills and sensory acuity (9,10). Many studies show that simulation-based training can teach and facilitate the technical aspects of a procedure and accelerate the learning curve (11). Different types of VR simulators are available. Simulators such as Lap Mentor or LapSim provide opportunities for novice surgeons to practice a procedure to proficiency before performing it in a patient. Lapsim® by Surgical Science (Gothenburg, Sweden) developed software with instruction and based on observations from the Copenhagen VATS surgeons and a standardized anterior approach. This simulation provides VATS lobectomy scenario for a right upper lobe with the dissection of hilum, vein, arteries and bronchus and stapling of vein, arteries, bronchus and fissures (10). The system was presented and tested at the 22nd meeting of the European Society of Thoracic Surgeons (ESTS) held in Copenhagen, Denmark in 2014.
Lap Mentor provides right upper lobectomy using an anterior approach as well. It allows the trainees to dissect and divide the vessels, bronchus and fissures. It can also teach how to manage complications such as injury of pulmonary artery, vein, phrenic nerve, pericardium and azygos vein (12).
Discussion
Residents and novice VATS surgeons are undergoing a paradigm change on how the future generation of thoracic surgeon learns VATS surgery. VATS surgery is gradually replacing open thoracotomy. VATS lobectomy for patients with early-stage NSCLC, compared with thoracotomy lobectomy is associated with less morbidity and improved overall survival rates (13). Learning and mastering it, is a challenging task for novice surgeons. Simulation creates an environment where novice surgeon’s masters psychomotor skills, sensory acuity and basic VATS knowledge outside the operating room, rather than spending time on operating room or “learning on patients” (3,10,11,13), which benefits patients and their safety.
Tedde et al. described the use of live swine for training surgeons in VATS lobectomies. In an advanced course on VATS procedure in Brazil, 40 swine were used for hands on course for left upper lobectomy in an anterior approach (8). They observed hypoventilation in 26 animals (65%), and 4 (10%) of them died in the last third part of surgery and 5% died due to bradicardy (8). Animal simulator provides a realistic environment for trainees but have practical and ethical challenges. Therefore, a number of bench top models were developed. Stupnik et al. presented an Ethicon Stupnik VATS simulator at the annual of the European Society of Thoracic Surgeons in Innsbruck 2017 (5). An artificial ribcage with disposable lung made from soft silicon, which was used to create four exercises of progressive difficulty. Each exercise addressed a core VATS skill, such as lung manipulation, wedge resection with energy device, wedge resection with endostapler and dissection of vessels. They concluded that simulators are an excellent tool for learning basic skills, which can be applied in the operating room during life procedure (6).
Jimenez et al. created a porcine heart and lungs blocks simulator for left upper lobectomy. These models were low of costs, the entire block costed around 2€, was easy to prepare and reproducible (14). They have developed a teaching program for trainees based on training session with simulation models. They recommend that surgical trainees must use simulation at least once per week, and they have the policy that trainees must complete 25 simulated lobectomies before starting VATS in real patients (14). Iwasaki et al. developed a simulation with circulating vessel in a lung, bronchus, which was covered with a plastic replica of human hemi thorax and was made of plastic. The cost of this simulation for a right upper lobectomy in total was around 300 dollars (15). This simulation may reduce the number of animal experiments, but the cost and preparation for each upper lobectomy is time consuming and expensive. Preparing such heart and lung blocks is time consuming and it will be expensive if each trainee should practice minimum once a week.
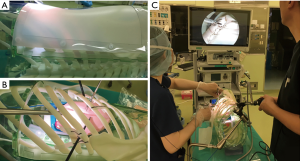
Sato and Morikawa created a realistic lung model from polyvinyl hydrogel which was inserted into an artificial human ribcage (7) (Figure 2). These simulators facilitate the training for trainees and create an almost realistic scenario for VATS surgeons. The disadvantages of biomimicking PVA model simulator is the cost, a unilateral model costs approximately 500 US dollars (7). With numerous issues surrounding animal and dry-lab simulators, the efficacy of these simulations is still under question.
Tong et al. evaluated validation of bench top simulation in thoracoscopic lobectomy. They included 31 residents with different level of experiences (12 experienced, 6 intermediates, and 13 novices) to perform left upper lobectomy in a porcine tissue simulator (16). The discriminative ability of the simulator was acceptable, and the authors concluded that it can be used as a tool for teaching for trainees and experienced surgeons.
Bjurström et al. investigated the effect of simulation-based training (6). They compared self-guided and educator-guided training. The study included surgeon group (n=10) and 30 randomized novice in three groups. All the groups trained for 3 hours on three scenarios before performing a wedge resection on a porcine lung. They concluded that training on simulation with educator enables novices to perform acceptable wedge resection in simulated model (6).
Black box has many advantages for trainees, such as low costs, few instruments, easily modified and enabling the realistic tissue feeling and giving a forced feedback, but in order to get feedbacks, the trainees need an instructor to observe the performance, it can be time consuming, expensive and the same procedure cannot be repeated again using the same tissue. VR simulations can create an environment where the novice VATS surgeons, can repeat the same procedure as many times as they want. They would get direct feedback without an instructor and realistic simulation of bleeding and anatomical variations are possible in the future. Other advantages of VR are easy access, streamlined training, and training outside working hours. Jensen et al. randomized 28 surgical residents to either a VR training on a nephrectomy or traditional black box simulator training (17). This study showed that the VR nephrectomy model did improve VATS performance over traditional black box simulator training and they concluded that dedicated VATS lobectomy software should be encouraged.
VR simulation
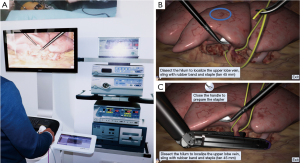
There are different VR simulators available in market now. Jensen et al. developed a VR simulator software LapSim, for a right upper lobectomy. Experienced surgeons worked with computer specialists and developed VATS lobectomy software for a VR simulator. The software was presented at 22nd meeting of European Society of Thoracic Surgeons (ESTS) in Copenhagen 2014. A total of 103 surgeons with different level of experiences, divided in three groups rated the simulator after a VATS lobectomy (Figure 3). The user’s realism of the VATS lobectomy was rated to a median of 5, on a scale of 1–7, with 7 being the best score (10).
The validity of simulation (LapSim®) was evaluated by Jensen et al. (18). Fifty-three participants from different countries with varying experiences in VATS lobectomy were included in the study. Several simulator metrics demonstrated significant differences between novices and experienced surgeons and pass/fail criteria for the test were set with acceptable consequences. They found high internal consistency for the metrics with a Cronbach’s alpha coefficient for standardized items of 0.91 (18).
Jensen et al. have also developed a novel assessment tool for evaluating competence in VATS lobectomy based on VATS experts’ consensus. A Delphi method used as a structured process for collecting and distilling knowledge from 31 international VATS experts. The VATSAT (VATS assessment tool) supports the learning of VATS lobectomy by providing structured feedback (19). This structured feedback system can also be used in learning of VATS simulation.
Solomon et al. provided a standard “gaming” laptop PC with a haptic feedback device to control surgical instruments. The simulation software is based on VR biomedical visualization developed for anatomic education. The system incorporates 3D animation and stores scientific data. This VR cognitive simulator can overcome deficiencies of existing training models (20).
Surgical simulation can facilitate a safe introduction into surgical practice (18). But what programs should be offered to novice surgeon? How the novice VATS surgeons should train in a simulation area, how many hours a day or a week? Are these simulations effective for training VATS surgeons? Can novice or experienced surgeons get acquainted, train and learn VATS surgery, when training in simulation? Is there an advantage of 3D over 2D?
Han et al. launched an endoscopic simulation program for uniportal surgery using a 3D video system. They concluded that a 3D video system has potential advantages, such as, improved procedure time and handling of instruments (21). Bagan et al. showed significant differences between 3D thoracic surgery versus 2D surgery. They included 18 patients and the time of procedure was 176 vs. 145 min with a P<0.001 (22).
Carrott et al. describes that advanced minimal-invasive procedures such as VATS require a specialized surgical skill set (23). There are key maneuvers and steps required to teach and learn VATS procedure. They advise that VR simulation can be a good starting point to gain some operative experience and then the porcine models would help the novice surgeons to develop a fine dissection skill and gain the “feel” for tissue strength (23).
Jensen et al. identified essential components of VATS upper right lobectomy to focus on simulation by a Delphi approach. Thirty-one surgeons participated and completed the study and 21 components were considered essential (19). Jensen et al. evaluated the competency in VATS lobectomy (24). Fifty-three participants performed two consecutive simulated VATS lobectomies in VR simulator, leaving 106 videos. Raters used VATSAT framework and the validity evidence was provided for a novel assessment tool for evaluating VATS lobectomy competence. They believe that VATSAT provides supervisors and assessors a structured approach for evaluating VATS lobectomy and aids to decide when the trainee is ready for unsupervised performance (11,24). We know that it’s a big step toward learning basic VATS skills on a simulator. The validity was demonstrated in a simulation environment (4). There is a need of a structured program for resident in thoracic surgery. The fundamental aspect is to contemplate how to teach and how to obtain autonomy that novice surgeons perform surgery in a safe environment. Divisi et al. suggest simulation should be a cornerstone for young trainee surgeons (25). It will be an irresponsible approach if the novice surgeons without any dexterity in basic movements can operate on patients (25). They describe that it would be a mistake to focus on VATS without having full mastery in open surgery. However, Konge et al. described that a novice surgeon with simulation-based training but limited experience in open surgery could achieve good VATS results under close supervision by experienced VATS surgeons (26).
Sandri et al. describe in their study, three steps from a trainee point of view and suggest that theses points could be of interest in setting-up a training program. (I) Stepwise approach to VATS lobectomy and standardization of teaching; where trainees get experience through small procedures, such as pleural biopsy, lung wedge resection etc. (II) Off-theatre independent training; simulation, like dry labs, VR simulations and (III) evaluation and certification should be seriously taken into account (27).
The Simulation Centre at Rigshospitalet, Copenhagen, Denmark, offers a “four-step approach” model to the medical training programs. These four steps include: (I) theoretical preparation; (II) on-site introduction to the simulation training assisted; (III) self-regulated practicing of the procedure; and (IV) end of simulation training certification (28).
Jiménez López et al. developed a training program in their institution, where the novice surgeons train on wet labs and all the procedures in the theatre have been recorded for discussion in regular training meeting and debriefing to evaluate times and skills (29).
Bedetti et al. described that VR training shorten the learning curve, even if it’s not designed to replace the experience gained in the operating theater. They evaluated skills with two sets, Objective Structured Assessment of Technical Skill (OSATS) and Global Operative Assessment of Thoracoscopic Skills (GOATS). Twenty voluntaries (trainees =12, consultants =8) completed tasks. Surgeons were evaluated for cognitive workload. They conclude that a VATS training curriculum with VR assessment is needed for trainees to train and learn VATS lobectomy techniques (30).
Fann et al. described in 2.5 days senior cardiothoracic surgeon’s symposium. They evaluated 12 simulators; six cardiac and six thoracic. Out of these six simulators, a VATS lobectomy simulator, porcine heart-ling block was created. Five surgeons evaluated its realism with scores of 2.2 to 2.8, where 1 was disagree 2, natural and 3 as agree (31).
Trehan et al. reviewed all the articles about simulation models applicable to cardiothoracic surgery to date. They described different types of simulators technologies such as simple bench models, virtual reality simulators, and Human performance simulator (32). They concluded that there was clear evidence for the unmistaken value of simulation. Simulation is believed to provide and serve as an important appurtenance for safer transition to better patient care and continued practice (32).
For novice and experienced surgeon, simulation has been characterized as reducing the technical learning curve and preparing surgeons for actual practice with improved patient safety. Simulators have the ability to provide trainees great practice outside the operating room (33).
The validity of assessment of competence has been discussed both in simulation and in the clinical setting and there is general believe that these newly developed assessment tools are beneficial in ensuring competency of future VATS surgeons and improves the safety of patients. Konge et al. recommend that all thoracic surgeons undergo mandatory VATS training, including simulation-based training must be emerged with training curriculum (34).
Conclusions
We have reviewed the recent literature on simulation. All the studies show that simulation has a valuable effect on learning VATS lobectomy. We are moving from an apprenticeship model to competency-based learning. Dry lab and wet-lab simulations offer many good opportunities, create an environment which novice thoracic surgeons, come closer to real procedures. It provides understanding and learning the VATS instruments, basic movements, but also poses a challenge regarding cost, preparation and its can be time consuming. Live animal creates a realistic environment, but cost, ethical and practical issues are important challenges.
VR allows trainees to practice the same procedure repeatedly while receiving feedback regarding their movements and progression. There is still a need to develop more software modules for VATS lobectomy, such as removal of all five lobes and handling of complications, e.g., bleeding from the pulmonary artery. We believe that VR may be a cornerstone for VATS thoracic training and simulation training should be implemented as part of VATS training in all centers around the world.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dominique Gossot) for the series “New Technologies for Advanced VATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2019.05.03). The series “New Technologies for Advanced VATS” was commissioned by the editorial office without any funding or sponsorship. RH Petersen: speaker fee from Medtronic. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bendixen M, Jorgensen OD, Kronborge C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomized controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Jensen K, Petersen RH, Hansen HJ. Video-assisted thoracic surgery lobectomy: Review of data strongly suggests the interest of its further implementation. European Journal of Clinical & Medical Oncology 2011;3:26-34.
- Petersen RH, Hansen HJ. Learning curve associated with VATS lobectomy. Ann Cardiothorac Surg 2012;1:47-50. [PubMed]
- Petersen RH, Gjeraa K, Jensen K, et al. Assessment of competence in Video Assisted Thoracoscopic Surgery (VATS) lobectomy. A Danish nationwide study. J Thorac Cardiovasc Surg 2018;156:1717-22. [Crossref] [PubMed]
- Stupnik T, Stork T. Training of video-assisted thoracoscopic surgery lobectomy: The role of simulator. Shanghai Chest 2018;2:52. [Crossref]
- Bjurström JM, Konge L, Lehnert P, et al. Simulation-based training for thoracoscopy. Simul Healthc 2013;8:317-23. [Crossref] [PubMed]
- Sato T, Morikawa T. Video-assisted thoracoscopic surgery training with a polyvinyl-alcohol hydrogel model mimicking real tissue. J Vis Surg 2017;3:65. [Crossref] [PubMed]
- Tedde ML, Filho FB, Belmonte EA, et al. Video-assisted thoracoscopic surgery in swine: an animal model for thoracoscopic lobectomy training. Interact Cardiovasc Thorac Surg 2015;21:224-30. [Crossref] [PubMed]
- de la Torre M, Rivas GD, Fernandez-Prado R, et al. Uniportal video-assisted thoracoscopic lobectomy in the animal model. J Thorac Dis 2014;6:S656-9. [PubMed]
- Jensen K, Bjerrum F, Hansen HJ, et al. A new possibility in thoracoscopic virtual simulation training: development and testing of a novel virtual reality simulator for video-assisted thoracoscopic surgery lobectomy. Interact Cardiovasc Thorac Surg 2015;21:420-6. [Crossref] [PubMed]
- Marshall MB. Simulation for technical skills. J Thorac Cardiovasc Surg 2012;144:S43-7. [Crossref] [PubMed]
- Available online: https://simbionix.com/lap-mentor-lobectomy-module
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for Early-Stage Non-Small Cell Lung Cancer: A Systematic Review of the Video-Assisted Thoracoscopic Surgery Versus Thoracotomy Approaches to Lobectomy. Ann Thorac Surg 2008;86:2008-16. [Crossref] [PubMed]
- Jimenez M, Gomez-Hernandez MT. Teaching video-assisted thoracic surgery lobectomy-using an ex vivo simulation model. J Vis Surg 2017;3:34. [Crossref] [PubMed]
- Iwasaki A, Okabayashi K, Shirakusa T. A model to assist training in thoracoscopic surgery. Interact Cardiovasc Thorac Surg 2003;2:697-701. [Crossref] [PubMed]
- Tong BC, Gustafson MR, Balderson SS, et al. Validation of thoracoscopic lobectomy simulator. Eur J Cardiothorac Surg 2012;42:364-9; discussion 369. [Crossref] [PubMed]
- Jensen K, Ringsted C, Hansen HJ, et al. Simulation-based training for the thoracoscopic lobectomy: a randomized controlled trial: virtual- reality versus black-box simulation. Surg Endosc 2014;28:1821-9. [Crossref] [PubMed]
- Jensen K, Bjerrum F, Hansen HJ, et al. Using virtual reality simulation to assess competence in video-assisted thoracoscopic surgery (VATS) lobectomy. Surg Endosc 2017;31:2520-8. [Crossref] [PubMed]
- Jensen K, Petersen RH, Hansen HJ, et al. A novel assessment tool for evaluating competence in video-assisted thoracoscopic surgery lobectomy. Surg Endosc 2018;32:4173-82. [Crossref] [PubMed]
- Solomon B, Bizekis C, Dellis SL, et al. Simulation video-assisted thoracoscopic lobectomy: a virtual reality cognitive task simulation. J Thorac Cardiovasc Surg 2011;141:249-55. [Crossref] [PubMed]
- Han KN, Kim HK, Choi YH. Application of a three-dimensional video system in the training for uniportal thoracoscopic surgery. J Thorac Dis 2018;10:3643-50. [Crossref] [PubMed]
- Bagan P, De Dominicis F, Hernigou J, et al. Complete thoracoscopic lobectomy for cancer: Comparative study of three-dimensional high definition with two-dimensional high definition video system. Interact Cardiovasc Thorac Surg 2015;20:820-3. [Crossref] [PubMed]
- Carrott PW Jr, Jones DR. Teaching video-assisted thoracic surgery (VATS) lobectomy. J Thorac Dis 2013;5:S207-11. [PubMed]
- Jensen K, Hansen HJ, Petersen RH, et al. Evaluating competency in video-assisted thoracoscopic surgery (VATS) lobectomy performance using a novel assessment too and virtual reality simulation. Surg Endosc 2019;33:1465-73. [Crossref] [PubMed]
- Divisi D, Barone M, Zaccagna G, et al. Video-assisted thoracoscopic surgery lobectomy learning curve: What program should be offered in a residency course? J Vis Surg 2017;3:143. [Crossref] [PubMed]
- Konge L, Petersen RH, Hansen HJ, et al. No extensive experience in open procedures is needed to learn lobectomy by video-assisted thoracic surgery. Interact Cardiovasc Thorac Surg 2012;15:961-5. [Crossref] [PubMed]
- Sandri A, Filosso PL, Lausi PO, et al. VATS lobectomy program: The trainee perspective. J Thorac Dis 2016;8:S427-30. [Crossref] [PubMed]
- Konge L, Ringsted C, Bjerrum F, et al. The Simulation Center at Rigshospitalet Copenhagen, Denmark. J Surg Educ 2015;72:362-5. [Crossref] [PubMed]
- Jiménez López M, Novoa Valentín NM. Implementing a VATS Lobectomy Program in Spain. The Wet lab, a Necessary Tool. Arch Bronconeumol 2016;52:579-80. [Crossref] [PubMed]
- Bedetti B, Bertolaccini L, Patrini D, et al. Virtual simulation and learning new skills in video-assisted thoracic surgery. Video-assist Thorac Surg 2018;3:35. [Crossref]
- Fann JI, Feins RH, Hicks GL, et al. Evaluation of simulation training in cardiothoracic surgery: The Senior Tour perspective. J Thorac Cardiovasc Surg 2012;143:264-72. [Crossref] [PubMed]
- Trehan K, Kemp CD, Yang SC. Simulation in cardiothoracic surgical training: Where do we stand? J Thorac Cardiovasc Surg 2014;147:18-24.e2. [Crossref] [PubMed]
- Nashaat A, Sidhu HS, Yatham S, et al. Simulation training for lobectomy: a review of current literature and future directions. Eur J Cardiothorac Surg 2018; [Epub ahead of print]. [PubMed]
- Konge L, Petersen RH, Ringsted C. Developing competency in video-assisted thoracic surgery (VATS) lobectomy. J Thorac Dis 2018;10:S2025-S2028. [Crossref] [PubMed]
Cite this article as: Haidari T, Konge L, Petersen RH. Simulation for the video-assisted thoracic surgery surgeon. Video-assist Thorac Surg 2019;4:12.