Nasal high-flow oxygen therapy during non-intubated thoracic surgery: does it offer a real advantage?
I read with enthusiasm the paper of Wang and colleagues (1) about the use of nasal high-flow oxygen therapy during non-intubated thoracoscopic surgery. The aim was to assess if high-flow oxygen therapy improved oxygenation and carbon dioxide wash out during non-intubated thoracic surgery.
Although the use of high-flow oxygen therapy had already been mentioned sporadically in non-intubated thoracic procedures (2), non-intubated surgery has been mainly performed in awake or non-awake spontaneously breathing patients with conventional facial mask, nasopharyngeal cannulas or laryngeal mask (3,4) (Figure 1). Several different ways of improving oxygenation have been reported but there is no standardized protocol and it’s center-dependent.
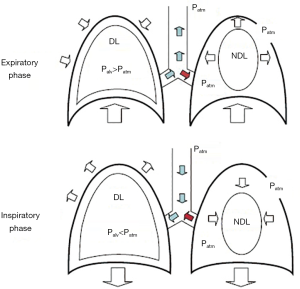
During non-intubated thoracic surgery, the risk of hypoxemia and hypercapnia are increased over conventional general anesthesia with tracheal intubation and one-lung mechanical ventilation (5). Once the iatrogenic surgical pneumothorax is stablished, a mismatch in the relation between ventilation and perfusion appears (6). Lung has its own protective mechanism, which is hypoxic pulmonary vasoconstriction, but using either volatile anesthetic drugs or intravenous agents like propofol may potentially inhibit this response (7). In addition, in non-intubated spontaneously breathing patients with iatrogenic surgical pneumothorax, a “rebreathing” phenomenon occurs (Figure 2), so exhaled air from the non-dependent collapsed lung (increased partial pressure of carbon dioxide and decreased in oxygen) introduces within the dependent lung, thus increasing the risk for hypoxemia and hypercapnia (9).
Although the authors had reported a non-negligible incidence of severe hypoxemia requiring conversion to general anesthesia in their huge series of non-intubated procedures (10), not more than 3% of all reported non-intubated procedures have required conversion to tracheal intubation due to this cause (1). In the other hand, severe hypercapnia has been described as a potentially fatal cause of conversion to tracheal intubation, especially in long lasting procedures such as pulmonary anatomical resections (lobectomies and segmentectomies). Severe hypercapnia has been underestimated in highest series and comparative studies of non-intubated major procedures, but in our experience, it is maybe the highest challenge to face while operating major procedures in spontaneously breathing patients. Risk factors for severe hypercapnia are respiratory depression due to deep sedation/general anesthesia, and the duration of the surgery, especially above 3 hours. The careful combination and titration of anesthetic drugs is essential to obtain a deep degree of sedation/general anesthesia while preserving spontaneous respiration, and some teams have claimed for the use of agents such as dexmedetomidine (11) in order to minimize the abolishment of respiratory stimulus.
Searching for a safe technique for both oxygenation and washing-out carbon dioxide in non-intubated surgery seems appealing, but it should always be kept in mind that in non-intubated procedures, appropriate lung collapse is advisable, so non-invasive ventilation (NIV) techniques may result counter-productive due to secondary lung insufflation, which makes more difficult some surgical steps.
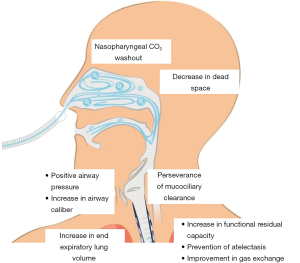
Nasal high flow (NHF) oxygen therapy, also called transnasal humidified rapid insufflation ventilator exchange (THRIVE) (12), delivers a humidified and heated combination of air and oxygen with flows up to 80 L/min and FiO2 between 0.21 and 1.0 (5). This flow loops around the soft palate and exits through the mouth, and meanwhile creates a highly turbulent supraglottic vortex (12) which constantly replenishes the pharynx with oxygen and prevents entrainment of room air. Its main benefits are a decrease in anatomical dead space, nasopharyngeal washout of carbon dioxide, maintenance of mucociliary clearance and increased end-expiratory lung volume. It also increases end expiratory transpulmonary pressure thus widening distal airway caliber and decreasing hydrostatic capillary-alveolar gradient (Figure 3). Due to this and to the high flows, a positive end expiratory pressure (PEEP) effect is achieved with values up to 5 cmH2O, so it creates a positive airway pressure that increases functional residual capacity, prevents atelectasis and improves gas exchange (13).
This means that NHF not only creates a kind of supraglottic reservoir of oxygen, but also has a ventilatory effect. Ventilation without ventilatory movements (oxygenation and CO2 clearance in the absence of lung ventilation) was first observed in 1909 by Samuel Meltzer during direct and continuous intratracheal oxygen insufflation (12). The limiting factor for this oxygenation technique is not oxygenation but increase in CO2 and decrease in blood pH, so risk for hypercapnia and acidosis.
Then, what benefits can NHF offer specifically for non-intubated thoracic surgery? Wang and colleagues conducted a comparative study with historical controls (1), which showed that NHF presented higher mean PaO2 preoperatively and during one-lung ventilation compared to oxygen mask (preoperative: 416.0 vs. 265.9 mmHg, P<0.01; one-lung breathing: 207.0 vs. 127.8 mmHg, P=0.01), and also a higher mean lowest oxygen saturation (98% vs. 95%, P=0.04) (1). The mean PaCO2 was not different in the NHF group compared to oxygen mask (preoperative: 48.0 vs. 46.2 mmHg, P=0.29; one-lung breathing: 49.4 vs. 49.6 mmHg; P=0.93). There were no differences in hospital stay and postoperative complications, and no mortality in both groups.
Authors hypothesized that the lack of differences between both groups in PaCO2 were probably secondary to the use of low flows in NHF (20 L/min) in order to avoid lung re-insufflation that occurred with high flows (70 L/min).
There are no randomized clinical trials comparing NHF with conventional oxygenation techniques in non-intubated thoracic surgery. This is probably the best attempt until date, although there are several inherent limitations due to the study design.
We observe that NHF is an effective technique for achieving excellent preoperative oxygenation and during one-lung ventilation, even though the drop in mean PaO2 is much higher with this technique from preoperative to one-lung ventilation than using oxygen mask (416.0 to 207.0 vs. 265.9 to 125.8 mmHg). However, is it necessary to get such a hyper oxygenated status? One could state that NHF is better for avoiding hypoxemia during non-intubated surgery but this conclusion wouldn’t be true: there was no episode of oxygen saturation lower than 90% in the NHF group (0%), in the other hand 8 of 30 patients (26.7%) in the oxygen mask group experienced oxygen saturation lower than 90%, but any of them lasted more than 5 minutes, and authors didn’t report statistical analysis for that comparison (rate of oxygen saturation lower than 90%). In fact mean lowest oxygen saturation in the oxygen mask group was 95%, which reflects oxygen mask was safe for oxygenation. There was no conversion to intubation in both groups, so there were no significant differences between the techniques in achieving a respiratory safe status during non-intubated surgery.
Although theoretically better for oxygenation (and even a kind of ventilation effect) than conventional oxygen masks, Wang and colleagues did not report cases of moderate or severe hypoxemia (PaO2 <60 mmHg) in any of the groups, and there was no conversion to intubation.
Mean PaCO2 were not significantly different between both groups, probably due to the low flows used to keep the lung deflated during one-lung ventilation: this reflects that despite NHF has shown a nasopharyngeal and main airway washout of carbon dioxide, this effect minimizes or even disappears when high flows (70–80 L/min) are not used.
Summarizing, NHF has proved to get higher mean PaO2 preoperatively and during one-lung ventilation, but there were no differences in severe hypoxemia neither conversion to intubation, and mean PaCO2 were similar between both techniques. There is no cost-effectiveness analysis in the mentioned study, but NHF would probably be more expensive than conventional oxygen mask. However, what appears even more important: there were no differences in the mean PaCO2, thus reflecting absence of improvement on carbon dioxide washout compared to conventional oxygen mask. I think that severe hypercapnia is probably one of the biggest challenges when performing non-intubated pulmonary anatomical resections due to the degree of sedation required and the length of the procedures, so future research should focus not so much on improving arterial oxygenation as on preventing severe hypercapnia.
Is there any way of getting a carbon dioxide washout effect while preserving lung collapse, without compromise for oxygenation? NHF therapy with intermediate flows might potentially show benefits in carbon dioxide washout without recruiting the non-dependent lung, but this should be studied in prospective randomized clinical trials. And some teams defend the use of laryngeal mask to ensure patency of the airway, keep spontaneous breathing while available for mechanical ventilation (14).
Leaving aside “last-resource” awake surgery for inoperable patients, I claim for a real attempt to standardize non-intubated procedures under deep sedation or general anesthesia in spontaneously breathing patients, and this requires randomized multicenter trials to shed light on a technique that nowadays remains in the dark for most thoracic surgeons, and whose pathophysiology still looks fuzzy.
Acknowledgments
To all who continuously seek for improvement and innovation in patient care, who never forget our primary objective (patient’s health), but never give up despite mistakes, critics and envy.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Video-Assisted Thoracic Surgery. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2019.03.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang ML, Hung MH, Chen JS, et al. Nasal high-flow oxygen therapy improves arterial oxygenation during one-lung ventilation in non-intubated thoracoscopic surgery. Eur J Cardiothorac Surg 2018;53:1001-6. [Crossref] [PubMed]
- Wang ML, Galvez C, Chen JS, et al. Non-intubated single-incision video-assisted thoracic surgery: a two-center cohort of 188 patients. J Thorac Dis 2017;9:2587-98. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, de la Torre M, et al. Single-port thoracoscopic lobectomy in a nonintubated patient: the least invasive procedure for major lung resection?. Interact Cardiovasc Thorac Surg 2014;19:552-5. [Crossref] [PubMed]
- Hung MH, Chan KC, Liu YJ, et al. Nonintubated thoracoscopic lobectomy for lung cancer using epidural anesthesia and intercostal blockade: a retrospective cohort study of 238 cases. Medicine (Baltimore) 2015;94:e727 [Crossref] [PubMed]
- Wittenstein J, Ball L, Pelosi P, et al. High-flow nasal cannula oxygen therapy in patients undergoing thoracic surgery: current evidence and practice. Curr Opin Anaesthesiol 2019;32:44-9. [Crossref] [PubMed]
- Lohser J. Evidence-based management of one-lung ventilation. Anesthesiol Clin 2008;26:241-72. v. [Crossref] [PubMed]
- Módolo NS, Módolo MP, Marton MA, et al. Intravenous versus inhalation anaesthesia for one-lung ventilation. Cochrane Database Syst Rev 2013;CD006313 [PubMed]
- Navarro-Martínez J, Gálvez C, Rivera-Cogollos MJ, et al. Intraoperative crisis resource management during a non-intubated video-assisted thoracoscopic surgery. Ann Transl Med 2015;3:111. [PubMed]
- Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery?. Eur J Cardiothorac Surg 2016;49:721-31. [Crossref] [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [Crossref] [PubMed]
- Klijian AS, Gibbs M, Andonian NT. AVATS: Awake Video Assisted Thoracic Surgery--extended series report. J Cardiothorac Surg 2014;9:149. [Crossref] [PubMed]
- Nouarei R, Shorthouse J, Keegan J, et al. Tubeless Ventilation – THRIVE. ENT and audiology news. Spotlight Innov 2018;27(2).
- Gustafsson IM, Lodenius Å, Tunelli J, et al. Apnoeic oxygenation in adults under general anaesthesia using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) - a physiological study. Br J Anaesth 2017;118:610-7. [Crossref] [PubMed]
- Irons JF, Martinez G. Anaesthetic considerations for non-intubated thoracic surgery. J Vis Surg 2016;2:61. [Crossref] [PubMed]
Cite this article as: Gálvez C, Navarro-Martínez J, Bolufer S, Sesma J, Galiana M. Nasal high-flow oxygen therapy during non-intubated thoracic surgery: does it offer a real advantage? Video-assist Thorac Surg 2019;4:8.