Two dimensional versus three dimensional video-assisted mediastinal lymphadenectomy: a meta-analysis
Introduction
On the attempt to reduce life-threatening postoperative morbidities in thoracic surgery, minimally invasive approaches, such as video-assisted thoracoscopic surgery (VATS), have been developed gaining wide acceptance worldwide due to their feasibility and safety as far as classical thoracotomy (1). However, conventional two-dimensional VATS approach carries certain disadvantages due to its technical features regarding optical resolution, pulmonary hilum visualization and issue concerning a flat two-dimensional camera rendering significantly affecting spatial resolution and dissection plane identification (2). The goniometric characteristics of the pleural cavity (sharpen and deep angles) and the relative axial size of a two-dimensional triangulation have represented not inconsiderable critical elements in the minimally invasive management of lung cancer since its dawn. This assumption appears to be rather corroborated in the mediastinal compartment approach. In fact, previous reports expressed some concerns about radicality of VATS node harvesting which is intimately linked to its oncological effectiveness and patients’ outcome in non-small cell lung cancer (NSCLC) (3,4). Beyond speculative and sometimes denied controversies, a video-assisted approach should ensure an accurate nodal staging for further therapeutic decisions without understating some potential complications such as bleeding and postoperative chylothorax, although some technical features in gaining access to some mediastinal spaces (i.e., paratracheal and subcarinal one) still argues debate (4,5). If on the one hand some limits seem to be overpassed by a proper learning curve or surgeons’ experience (6), on the other some technical limitation can influence surgical field visualization, view ranges and the exposition of structures (7). Some of these aspects seems to be overpassed with the adoption of three-dimensional technology in video-assisted procedures which are characterized by a magnified and deeper surgical field visualization due its stroboscopic visual perception allowing to reduce “blind” areas and by a high degrees of freedoms with a looking-around-the-corner-view facility resulting in an excellent spatial and hand-eye coordination (8) and making surgical dissection easier than in 2D visual rendering. Basing on this discussed topic and the theoretical effects on hilar and mediastinal node management in NSCLC patients, 3D- and conventional VATS dissections have been investigated through a systematic review and meta-analysis by evaluating their pooled effects on both oncological aspects and postoperative incidence of chylothorax according to these techniques.
Methods
Study design
A PubMed Embase, Google Scholar, OpenDOAR research was carried out by two investigators from the authors’ panel in order to assay relevant and suitable report published up to Aug 31, 2018, according to the following Boolean function of medical subject heading (MeSH) terms: ((((((((three-dimensional) OR 3D) AND two-dimensional) OR 2D) AND video-assisted thoracic surgery) OR VATS) AND lymphadenectomy) OR node staging) OR lymphatic staging)))))))). The last search was run on October 25, 2018. All potential eligible articles were reviewed according to a two-phase process (title-abstract and full-text evaluation) and the following inclusion criteria: (I) video-assisted thoracoscopic lymph node dissection in NSCLC or esophageal cancer patients; (II) cohort analysis between two- and three-dimensional harvesting; (III) clearly description of surgical techniques and technological instrumentation; (IV) report of an exhaustive exploration of the mediastinal groves as far as hilar compartment; (V) proper definition of the mean number of lymph node harvested; (VI) report of patients’ surgical outcome, especially concerning bleeding and postoperative chylothorax and finally; (VII) article written only in English. Only retrospective monocentric or multicentric studies and prospective randomized-controlled trials (RCTs) were considered, the remaining ones (i.e., review, states of art, case reports and editorials) were excluded as their poor statistical relevance. Black-box, experimental model or geometrical analysis was also excluded. The remaining eligible reports were further analysed for data extraction by two independent reviewers to derive the following informations: authors, year of publication, country of publication, enrollment period, number of patients, primary disease, inclusion criteria (if reported), lymph node harvesting technique, devices or instrumentations and patients’ outcome.
Endpoints
The primary endpoint for this meta-analysis was to assess any superiority of 3D-VATS lymph node dissection over conventional 2D-VATS.
Secondary endpoints included:
- Video-assisted technology vs. paratracheal (stations #2–4) and subcarinal (station #7) dissection;
- Postoperative blood loss;
- Postoperative chylothorax.
Statistical analysis
The meta-analysis was conducted with Microsoft Excel 2016 (Microsoft®, Redmond, USA). All data were collected as absolute numbers (N), percentages (%), mean and standard deviation (SD) with their relative 95% confidence interval (95% CI). Statistical differences or correlations between groups were analysed according to the paired t-test both for categorical and continuous variables. For each endpoint, a summarized Forrest plot according was derived according to their mean difference (mean diff.), standard error (SE), 95% CI, t-statistic value or Chi-squared value (for proportions) and difference freedom (DF). A P value <0.05 was considered statistically significant.
Results
Data extraction process
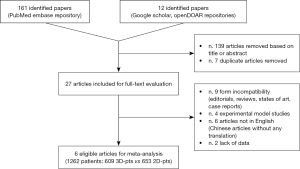
According to the adopted Boolean function, one hundred sixty-one papers were identified. Further twelve articles were included after other repositories investigation. Thereafter, one hundred thirty-nine were removed based on title or abstracts. Concerning the remaining twenty-seven potentially relevant articles, a second-phase analysis was carried out throughout a full-text evaluation. Only six articles resulted eligible for meta-analysis (9-14) (Table 1). In particular twenty-one were excluded due to: (I) form incompatibility (nine articles); (II) experimental model studies (four articles); (III) articles written in other languages than English and lacking of any translation (six articles); and (IV) lack of data making unsuitable extraction process. At the end of the analysis, 1,262 patients were enrolled for the study (609 3D-VATS patients and 653 2D-VATS patients) (Figure 1).
Full table
Studies’ quality assessment
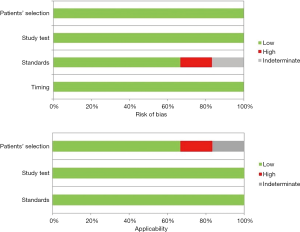
Quality analysis was carried out through the QUADAS-2 criteria panel, as reported in Table 2. Any source of bias was investigated by two reviewers and resulting in a high risk for selection bias according to standards in one article, whilst data in another one protocol were unclear. Similar issues were found evaluating applicability for both studies, as a result of an unclear patients’ selection otherwise being enrolled for stage I to IV NSCLC (Table 2) (Figure 2). No resumable data could be assessed about surgeons’ experience or skills in video-assisted approach, although high-volume centres were only enrolled.
Full table
Lymph node management technique
All the studies elected for analysis clearly reported lymph node harvesting technique. In particular each patient, both in the two-dimensional and three-dimensional arms, underwent radical mediastinal node dissection (9-14). Concerning with lung cancer, hilar stations (#10, #11) were included, while an en bloc resection with surgical specimen was reported in case of esophageal neoplasms (9,11). However, the Authors did not report their technique about the adoption of any energy device or any instrumentation for dissection.
Surgical and oncological data (Figures 3,4)
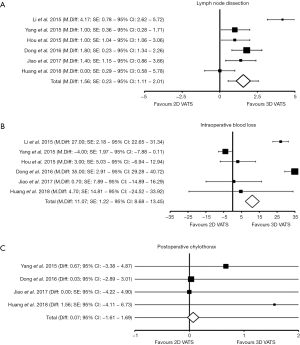
Number of harvested lymph nodes
The number of harvested lymph nodes for each cohort of patients was clearly reported in all six elected studies, accounting 1,262 patients (3D patient vs. 2D patients: 609 vs. 653). At the weighted-pooled analysis, no significant cumulative effect was found (7.37% vs. 23.77% vs. 12.20% vs. 28.53% vs. 13.07% vs. 15.06%, P=0.694). With a mean number of dissected nodes of 18.62±3.53 and 17.06±4.47, respectively, there was a significant difference between cohorts (mean difference: 1.56; SE: 0.23; 95% CI: 1.11–2.01, DF=1,260; t=6.85; P<0.001) favouring a three-dimensional approach (Tables 3,4) (Figure 3A).
Full table

Full table
Paratracheal and subcarinal node dissection
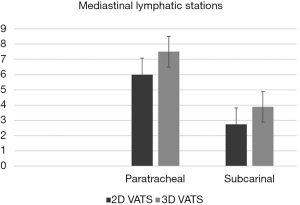
Two studies reported influence of three-dimensional technology for specific mediastinal node areas. In particular, at the pooled analysis, no significant benefits were found to gain access to paratracheal compartment on the aspect of the mean number of dissected lymph nodes (#2–4 3D vs. 2D VATS: P=0.619). Otherwise, significant difference between technology was found by considering the subcarinal region (#7 3D vs. 2D VATS: P=0.005) (Figure 4).
Intraoperative blood loss
Intraoperative blood-loss estimation was reported in all the included studies, accounting 1,262 patients (3D patient vs. 2D patients: 609 vs. 653). At the weighted-pooled analysis, no significant cumulative effect was found (7.37% vs. 23.77% vs. 12.20% vs. 28.53% vs. 13.07% vs. 15.06%, P=0.694). With a mean loss of 106.38±23.78 mL in the 3D group and of 117.45±19.30 in the conventional one, there was a statistical significant difference between cohorts (mean difference: 11.07; SE: 1.22; 95% CI: 8.68–13.45, DF=1,260; t=9.10; P<0.001) (Tables 3,4) (Figure 3B).
Postoperative chylothorax
Postoperative chylothorax was reported in four studies, enrolling 1,015 patients (3D patient vs. 2D patients: 486 vs. 529). At the weighted-pooled analysis, no significant cumulative effect was found (29.56% vs. 35.47% vs. 16.26% vs. 18.72%, respectively; P=0.483). With an incidence of 1.44% and 1.51% of chyle leak for 3D-and 2D patients, no augmented risks were found according to technological supply (percentage difference: 0.07; χ2=0.009; 95% CI: −1.61–1.69, DF=1; P=0.926) (Tables 3,4) (Figure 3C).
Discussion
Two-dimensional VATS carries several disadvantages due to technical limits and anatomical issues. Concerning with instrumentation, the visual constraints as far as the lack of stereo-perception and difficulties in hand-eye coordination could significantly influence surgeons’ performance and dissection phases in the operative theatre (15). Moreover, the undeniable geometric features of the thoracic cavity with reduced spaces and freedom grades of movements contribute to making uncomfortable and difficult some maneuvers that, only with an adequate learning curve and by means of expedients, may be accomplished. The absence of depth perception and spatial orientation have been recently countered by three-dimensional systems with an accuracy comparable to open surgery by the adoption of visual performance and motor skills resulting in an improved discrimination and recognition of targeted organs or regions (16,17). However, this technology is far to be widely accepted due to its high expensiveness and the requirement of dedicated operative setting (18). Moreover, notwithstanding health expenditures and economic impacts, last generation 3D devices ensure several and not negligible benefits by reducing black-angle maneuvers and thus increasing patients’ safety. An exhaustive and radical lymph node assessment is a cornerstone for the surgical management of both lung and esophageal cancer as being the most important prognostic factor for patients’ outcome (19-21). In particular, metastatic nodes influence prognosis as absolute number of harvested glands and as their ratio upon N0 ones (22,23). Thereafter, every attempt to improve surgical dexterity and precision movements has to be advocated and in particular to gain access to some tedious mediastinal regions, such as the paratracheal and the subcarinal ones. In these lymph node stations a conventional flat vision could limit dissection and so oncological radicality in face of not negligible augmented risks of intraoperative complications from blind areas, such as bleedings. Speculating about surgical data and the effects of innovative stroboscopic resources with conventional video-assisted approaches, the mean number of harvested hilar and mediastinal lymph nodes significantly increased in the three-dimensional cohort (3D- vs. 2D-group: 18.62±3.53 vs. 17.06±4.47, 95% CI: 1.11–2.01, P<0.001) confirming feasibility of 3D lymphadenectomy. Differences could be explained upon the above mentioned technical properties allowing a safer and so a more extensive dissection among planes as far as an increased surgeon’s safety to overpass beyond threatening fears to face with sudden complications. This aspect seem to be confirmed at the analysis of the subcarinal node dissection where a three-dimensional approach seems to ameliorate space assessment with an increased mean number of harvested node (3D- vs. 2D- VATS: 3.89 vs. 2.73, P=0.005), as reported by Li et al. (9). In contrast, augmented reality does not seem to interfere with paratracheal space dissection (3D- vs. 2D-VATS: 7.50 vs. 6.00, P=0.619) and, as reported by Huang et al. (14), reasons should be found in anatomical issues rather than in technical aspects, as the region located in the upper mediastinum and not disturbed by the sequences of pulmonary lobectomies. Moreover, paratracheal dissection classically is carried out at the end of the procedure with the surgical specimen extracted without spatial encumbrance and also a flat visualization of the surgical field allows the recognition of the anatomical landmarks above and below the azygos vein. In regard to intraoperative bleedings, a 3D approach significantly reduces losses (3D-vs. 2D-VATS: 106.38±23.78 vs. 117.45±19.30; 95% CI: 8.68–13.45, P<0.001) justifying a more accurate dissection and haemostasis of planes, especially around vascular structures. In particular, this result should be corroborated only by technical aspects and by an increased dexterity in movements making a three-dimensional endoscopic technique closer to telerobotics rather than to conventional VATS (18). Another not negligible aspect to be considered is the risk of postoperative chylothorax, when facing lymphatics and their dissection. Historically, chyle leaks represent potentially serious complication of thoracic surgical procedures, especially after esophageal surgery with a cumulative incidence ranging from 0.5% and 4% (24-26), usually secondary to lymphatic cauterization failure, direct channel disruption or thoracic duct injuries. From our analysis, notwithstanding visualization and definition properties, last generation video-assisted strategies do not reduce its incidence, as being almost irrelevant (3D- vs. 2D-VATS: 1.44% vs. 1.51%, 95% CI: −1.61–1.69, P=0.926). In fact, putative causes should be traced on sealing or clipping techniques as far as proper dissection rather than the possibility to magnify surgical field and calling in the surgeon’s accuracy and dexterity.
Limits of the study
Although a systematic approach, our evidences should be interpreted in the context of some limitations. In the first, most of eligible articles presented a low sample size and only two of them enrolled more than three hundred patients. In the second, no data about surgeons’ experience as far as the adoption of any energy device for dissection may be assessed. In the third, most of studies were retrospectively and single-center constructed. Moreover, most of them were strictly designed with ineluctable inclusion criteria clearly described. However, due to these aspects, an undeniable possibility of type 2 error should be considered.
Conclusions
Three-dimensional video-assisted approach in the surgical management of pulmonary or esophageal cancers ensures a better management of lymphatics and of their dissection with theoretical benefits both on short-term (bleedings) and oncological outcomes. New devices have overpassed historical limits of celioscopic procedures and, nowadays, seem to be closer to the fitness of open surgery rather in the past.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dominique Gossot) for the series “New Technologies for Advanced VATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2019.02.01). The series “New Technologies for Advanced VATS” was commissioned by the editorial office without any funding or sponsorship. RC serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Sep 2017 to Aug 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Anonymous data from published articles.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Pardolesi A, Park B, Petrella F, et al. Robotic anatomic segmentectomy of the lung: technical aspects and initial results. Ann Thorac Surg 2012;94:929-34. [Crossref] [PubMed]
- Mathisen DJ. Is Video-Assisted Thoracoscopic Lobectomy Inferior to Open Lobectomy Oncologically? Ann Thorac Surg 2013;96:755-6. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-353; discussion 353. [Crossref] [PubMed]
- Denlinger CE, Fernandez F, Meyers BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg 2010;89:1730-5; discussion 1736.
- Wang H, D’Amico TA. Efficacy of mediastinal lymph node dissection during thoracoscopic lobectomy. Ann Cardiothorac Surg 2012;1:27-32. [PubMed]
- Wang W, Yin W, Shao W, et al. Comparative study of systematic thoracoscopic lymphadenectomy and conventional thoracotomy in resectable non-small cell lung cancer. J Thorac Dis 2014;6:45-51. [PubMed]
- Sahu D, Mathew MJ, Reddy PK. 3D Laparoscopy - Help or Hype; Initial Experience of A Tertiary Health Centre. J Clin Diagn Res 2014;8:NC01-03. [PubMed]
- Li Z, Li JP, Qin X, et al. Three-dimensional vs. two-dimensional video assisted thoracoscopic esophagectomy for patients with esophageal cancer. World J Gastroenterol 2015;21:10675-82. [Crossref] [PubMed]
- Yang C, Mo L, Ma Y, et al. A comparative analysis of lung cancer patients treated with lobectomy via three-dimensional video-assisted thoracoscopic surgery versus two-dimensional resection. J Thorac Dis 2015;7:1798-1805. [PubMed]
- Hou Y, Guo W, Yang Z, et al. Comparative study of 3D thoracoscopic esophagectomy versus 2D thoracoscopic esophagectomy for esophageal carcinoma. Zhonghua Wei Chang Wai Ke Za Zhi 2015;18:889-92. [PubMed]
- Dong S, Yang XN, Zhong WZ, et al. Comparison of three-dimensional and two-dimensional visualization in video-assisted thoracoscopic lobectomy. Thorac Cancer 2016;7:530-4. [Crossref] [PubMed]
- Jiao P, Wu QJ, Sun YG, et al. Comparative study of three-dimensional versus two-dimensional video-assisted thoracoscopic two-port lobectomy. Thorac Cancer 2017;8:3-7. [Crossref] [PubMed]
- Huang W, Liu J, Liang W, et al. Outcome and Safety of Radical Resection in Non-Small Cell Lung Cancer Patients via Glasses-Free 3-Dimensional Video-Assisted Thoracoscope Versus 2-Dimensional Video-Assisted Thoracoscope. Surg Innov 2018;25:121-7. [Crossref] [PubMed]
- Wilhelm D, Reiser S, Kohn N, et al. Comparative evaluation of HD 2D/3D laparoscopic monitors and benchmarking to a theoretically ideal 3D pseudodisplay: even well-experienced laparoscopists perform better with 3D. Surg Endosc 2014;28:2387-2397. [Crossref] [PubMed]
- Yamauchi Y, Shinohara K. Effect of binocular stereopsis on surgical manipulation performance and fatigue when using a stereoscopic endoscope. Stud Health Technol Inform 2005;111:611-4. [PubMed]
- Blake R, Levinson E. Spatial properties of binocular neurones in the human visual system. Exp Brain Res 1977;27:221-32. [Crossref] [PubMed]
- Divisi D, Barone M, Crisci R. Three-dimensional video-assisted thoracic surgery for pulmonary resections: an update. J Vis Surg 2017;3:79. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming(Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Veeramachaneni NK, Zoole JB, Decker PA, et al. Lymph node analysis in esophageal resection: American College of Surgeons Oncology Group Z0060 trial. Ann Thorac Surg 2008;86:418-21; discussion 421. [Crossref] [PubMed]
- Alline M, Bertrand MM, Colombo PE, et al. Lymph node dissection: what for? From esophagus to rectum: surgical and lymph node related prognostic factors. Bull Cancer Paris 2014;101:368-72. [PubMed]
- Ou SH, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J Thorac Oncol 2008;3:880-6. [Crossref] [PubMed]
- Li Q, Zhan P, Yuan D, et al. Prognostic value of lymph node ratio in patients with pathological N1 non-small cell lung cancer: a systematic review with meta-analysis. Transl Lung Cancer Res 2016;5:258-64. [Crossref] [PubMed]
- Martucci N, Tracey M, Rocco G. Postoperative Chylothorax. Thorac Surg Clin 2015;25:523-8. [Crossref] [PubMed]
- Shah RD, Luketich JD, Schuchert MJ, et al. Postesophagectomy Chylothorax: Incidence, Risk Factors, and Outcomes. Ann Thorac Surg 2012;93:897-903; discussion 903-4.. [Crossref] [PubMed]
- Miao L, Zhang Y, Hu H, et al. Incidence and management of chylothorax after esophagectomy. Thorac Cancer 2015;6:354-8. [Crossref] [PubMed]
Cite this article as: Divisi D, Barone M, Zaccagna G, Crisci R. Two dimensional versus three dimensional video-assisted mediastinal lymphadenectomy: a meta-analysis. Video-assist Thorac Surg 2019;4:5.