
Surgical simulation in robotic-assisted thoracic surgery: training
Introduction
Minimally invasive thoracic surgery witnessed exponential growth when the Food and Drug Administration approved robotic approaches for thoracic surgery in 2000. Institutions began reporting single center experiences with robotic thoracic surgery via the da Vinci Surgical System (Intuitive Surgical; Sunnyvale, CA) (1-3). Studies have shown the robot is safe and efficacious with equitable outcomes compared to traditional thoracoscopic approaches (4-9). Since the inception of robotic assisted thoracoscopic surgery debate exists regarding the utility of robotic approaches related to cost and value, however, the application to thoracic procedures continues to expand from mediastinal resections to lobectomies.
Surgical training in thoracic surgery now challenges trainees to master open and minimally invasive approaches. There remains heterogeneity and limited exposure in robotic training compared to traditional video-assisted thoracic surgery approaches (8). Recent graduates sitting for the American Board of Thoracic Surgery in 2014 reported the need for more exposure and confidence in robotic pulmonary operations (55.8%) (10). Strategies to improve training, specifically among robotic-assisted thoracic surgery remain an area of interest needing continued advancement and standardization. Simulation has been proposed as a catalyst for such skill proficiency and training.
Simulation
Simulation has been well described as a necessary training entity prior to live robotic surgery (11,12) and should be approached in a step-wise fashion both during dry/virtual simulation and during live surgery (8,12,13). Robotic dual console systems facilitate direct supervision and step-wise transfer of instrumentation control between the mentor and trainee in a guarded and safe fashion once competency has been demonstrated (8). There are four commercially available robotic simulators that currently exist including the da Vinci Skills Simulator (dVSS; Intuitive Surgical, Sunnyvale, CA, USA), the mimi dV-Trainer (dV-Trainer; Mimic Technologies, Inc, Seattle, WA, USA), the Robotic Surgical Simulator (RoSS; Simulated Surgical Systems, Buffalo, NY, USA), and the Sim-Surgery Educational Platform (SEP; SimSurgery, Norway). Robotic surgical simulators demonstrate robotic skill and can be utilized to improve basic robotic performance through proficiency-based training (14-19). While existing simulators focus on fundamental robotic skill development and proficiency through basic tasks and procedures, the dVSS features a thoracic-specific procedure simulator for lobectomy (Figure 1). The module features step-by-step guidance to perform a robotic lobectomy with post-procedure feedback including time and economy of movement in addition to safety and complication metrics.

As with minimally invasive approaches such as video-assisted thoracic surgery, robotic surgery has unique learning curves that must be achieved. Institutional studies suggest that approximately 20 robotic lobectomies performed by surgeons with video-assisted thoracic surgery experience are needed to gain appropriate proficiency regarding patient outcomes based on operative time, morbidity, mortality, conversion rate, length-of-stay, and surgeon comfort (21-24). Di Lorenzo et al. showed that novice trainees without prior thoracoscopic experience could learn basic robotic maneuvers and complete rudimentary tasks (25). Meanwhile, seasoned surgeons found the console intuitive and adapted more quickly than junior surgeons despite demonstrating initial difficulties adapting to the robotic system. A 4-week robotic training protocol improved bimanual carrying, needle passing, and suture tying among medical students with no prior open or minimally invasive operative experience (26). Shorter learning curve times for robotic thoracic surgery compared to video-assisted thoracic surgery has been reported (27). It remains difficult to compare transitions from open thoracotomy to first video-assisted thoracic surgery from a surgeon who has mastered video-assisted thoracic surgery to transition to first robotic-assisted thoracic surgery (28). A paucity of literature exists; nonetheless, data suggests both novice and experienced surgeons with or without prior minimally invasive exposure can achieve robotic proficiency through robotic simulation.
Approaches to robotic thoracic-procedure proficiency have been detailed highlighting a suggested stepwise progression in case complexity with level 1, 2, and 3 classifications (12). Level 1 operations may include mediastinal resection, lymph node dissection, wedge lobe resections, sympathotomy, and/or pleural biopsy or resection. Level 2 procedures are suggested to include thymectomy, inferior mediastinal resections, diaphragm plication, mid-esophageal resection, esophagectomy without anastomosis, and chest wall resection. Level 3 operations gain complexity such as segmentectomy, lobectomy, sleeve resection, foregut/myotomy, and Ivor Lewis esophagectomy with anastomosis. Robotic skill proficiency can be measured on dry simulation “check off” and robotic simulator modules to signal when surgeons possess the necessary fundamental skills for live surgery. Nonetheless, there remains a paucity of policy that mandates standardized proficiency levels for credentialing robotic-assisted thoracic surgery and currently exists at the institutional level (19,29).
Simulation program essentials
With recent time constraints on surgical education, the role of simulation has become paramount to provide the additional exposure and opportunities to hone skill and performance levels to achieve the necessary proficiencies and learning curve. As such, a vast array of robotic simulation curriculums and modules are cited in the literature, however, calls for universal standardized curriculums are resounding. Standardized curriculums and tools to measure proficiency applicable to any simulator are needed. The Institute for Surgical Excellence highlights a curriculum through the Robotic Training Network focused on robotic overviews and background, pre- and post-test knowledge assessments, introduction to robotic systems, bedside assistant, console surgeon, team training and communication, and specialty-specific education and training (30). The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) proposed a Fundamentals of Robotic Surgery curriculum funded through the Department of Defense (31). Their course includes an introduction to surgical robotic systems, didactic instructions for robotic surgery systems, psychomotor skills curriculum, and team training and communication skills. Additionally, the American College of Surgeons and the Association of Program Directors in Surgery Surgical Skills Curriculum for Residents proposed a general curriculum encompassing 20 modules addressing basic surgical skills, 15 modules focused in advanced skills and procedures, and 10 modules addressing team-based/non-technical skills (32). This general curriculum served as the framework for a recent Delphi panel consensus for the design, validation, and implementation of a simulation-based curriculum (33).
Robotic surgical education should include didactics, observation, and proficiency check-off through simulation and live performance (Table 1). Surgeons and their operating team should attend certified didactic training courses (12,34). A consensus group with leadership from the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) and the Minimally Invasive Robotic Association assembled in 2006 to provide standardized comments to robotic training (35). Didactics should cover robotic technology, device function, room setup, bedside assistance/technique and ability to trouble-shoot complications. In addition, procedure specific indications, patient workup, and operative planning not limited to trochar and instrumentation placement are necessary. Case observation should focus on patient preparation and positioning, robotic system setup, and intraoperative procedure-specific technical pearls. Simulation allows for surgeons to become familiar with the robot and achieve skill proficiency before entering the operative theatre.
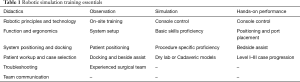
Full table
In 2016, an international consensus group from 14 institutions in 8 countries performed an evaluation of robotic thoracic surgery training through an expert Delphi Panel (36). A panel created by members of the European Society of Thoracic Surgeons (ESTS) and European Association for Cardiothoracic Surgery (EACTS) performed a systematic review of current evidence for training in robotic thoracic surgery and completed surveys through Delphi process to formulate recommendations for a standardized curriculum. Staged learning pathways to segment and clearly define curriculum modules are advantageous and should start with didactics similar to those proposed in Table 1. Didactics and e-learning questions should focus on robotic equipment, patient selection, docking and port placement, troubleshooting, emergency management and conversion, and team communication. Team training, included in didactics and observation, should include bedside assistance, docking, emergency scenarios, team decision-making, and efficient robotic room turnover. Training should focus on non-technical development and evaluation in situation awareness, decision-making, communication, and teamwork/leadership. These performance evaluations satisfy current Accreditation Council for Graduate Medical Education (ACGME) competencies such as professionalism, systems-based practice, interpersonal and communication skills. They recommend certified thoracic surgeons serving as mentors to proctor trainees at accredited centers recognized by a society of thoracic surgeons at institutions with more than 50 robotic thoracic cases per year.
While these curriculums are not unique to thoracic surgery, they provide a framework for the design and distribution of a universal thoracic training program. It is well recognized that standardized procedures and operative schemes are necessary to perform robotic-assisted thoracic surgery (37). While institutions have published on unique experiences with robotic surgical training, a consensus curriculum from the above-mentioned research is yet to be recognized by the Society of Thoracic Surgeons (STS). The STS currently sponsors symposiums on robotic thoracic surgery training for surgeons interested in program-start up and continued medical education.
Future direction
While consensus statements regarding robotic training curriculums are available based on expert opinions, comparison studies evaluating performance and effectiveness of these curriculums are needed to form a consensus on best practices regarding surgical simulation in thoracic surgery (14). A standardized curriculum will be adopted and applied to universal credentialing in robotic thoracic surgery, however, it will take time for institutions to integrate universal credentialing given current culture and policies. As simulators advance, surgeons will be able to rehearse approaches and simulate outcomes to complex cases based on procedure-specific simulation rendered from patient-specific anatomy acquired through computed tomography imaging (35). Such approaches and theory are under development in China and Japan using 3D-printed models and imaging to plan and assist live thoracoscopic surgical approaches in segmentectomy, however, this has not been applied to robotic simulators to our knowledge (38,39).
Conclusions
A universal robotic training curriculum in thoracic surgery is needed for effective standardized training, certification and credentialing. The curriculum should be robust, multifaceted including didactics, observation, simulation, and hands-on performance.
Acknowledgments
The photographic images and videos are provided here exclusively for promotion, editorial, educational and/or media coverage of Intuitive Surgical and its products. This notification serves as an authorization to make duplicate copies of the available high-resolution scans for these uses only.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Yoshihisa Shimada) for the series “Current Status of Surgical Simulation in Video-assisted Thoracic Surgery and Robot-assisted Thoracic Surgery” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.10.04). The series “Current Status of Surgical Simulation in Video-assisted Thoracic Surgery and Robot-assisted Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Morgan JA, Ginsburg ME, Sonett JR, et al. Thoracoscopic lobectomy using robotic technology. Heart Surg Forum 2003;6:E167-9. [PubMed]
- Bodner J, Wykypiel H, Wetscher G, et al. First experiences with the da Vinci operating robot in thoracic surgery. Eur J Cardiothorac Surg 2004;25:844-51. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. [Crossref] [PubMed]
- Ninan M, Dylewski MR. Total port-access robot-assisted pulmonary lobectomy without utility thoracotomy. Eur J Cardiothorac Surg 2010;38:231-2. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Wei B, D'Amico TA. Thoracoscopic versus robotic approaches: advantages and disadvantages. Thorac Surg Clin 2014;24:177-88. vi. [Crossref] [PubMed]
- Kumar A, Asaf BB. Robotic thoracic surgery: The state of the art. J Minim Access Surg 2015;11:60-7. [Crossref] [PubMed]
- Chu D, Vaporciyan AA, Iannettoni MD, et al. Are There Gaps in Current Thoracic Surgery Residency Training Programs? Ann Thorac Surg 2016;101:2350-5. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Starting a robotic program in general thoracic surgery: why, how, and lessons learned. Ann Thorac Surg 2011;91:1729-36; discussion 1736-7.
- Cerfolio RJ, Bryant AS. How to teach robotic pulmonary resection. Semin Thorac Cardiovasc Surg 2013;25:76-82. [Crossref] [PubMed]
- Linsky PL, Wei B. Training in robotic thoracic surgery. J Vis Surg 2018;4:1. [Crossref] [PubMed]
- Bric JD, Lumbard DC, Frelich MJ, et al. Current state of virtual reality simulation in robotic surgery training: a review. Surg Endosc 2016;30:2169-78. [Crossref] [PubMed]
- Walliczek-Dworschak U, Mandapathil M, Fortsch A, et al. Structured training on the da Vinci Skills Simulator leads to improvement in technical performance of robotic novices. Clin Otolaryngol 2017;42:71-80. [Crossref] [PubMed]
- Moglia A, Ferrari V, Morelli L, et al. A Systematic Review of Virtual Reality Simulators for Robot-assisted Surgery. Eur Urol 2016;69:1065-80. [Crossref] [PubMed]
- Hung AJ, Zehnder P, Patil MB, et al. Face, content and construct validity of a novel robotic surgery simulator. J Urol 2011;186:1019-24. [Crossref] [PubMed]
- Willis RE, Van Sickle KR. Current Status of Simulation-Based Training in Graduate Medical Education. Surg Clin North Am 2015;95:767-79. [Crossref] [PubMed]
- Schreuder HW, Wolswijk R, Zweemer RP, et al. Training and learning robotic surgery, time for a more structured approach: a systematic review. BJOG 2012;119:137-49. [Crossref] [PubMed]
- Wahl TS, Wei B. da Vinci Skills Simulator (Intuitive Surgical, Sunnyvale, CA, USA) showing segment of the lobectomy module—right upper lobectomy with vessel transection using a robotic stapler. Asvide 2018;5:852. Available online: http://www.asvide.com/article/view/28232
- Meyer M, Gharagozloo F, Tempesta B, et al. The learning curve of robotic lobectomy. Int J Med Robot 2012;8:448-52. [Crossref] [PubMed]
- Meier M, Horton K, John H. Da Vinci(c) Skills Simulator: is an early selection of talented console surgeons possible? J Robot Surg 2016;10:289-96. [Crossref] [PubMed]
- Cheufou DH, Mardanzai K, Ploenes T, et al. Effectiveness of Robotic Lobectomy-Outcome and Learning Curve in a High Volume Center. Thorac Cardiovasc Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Reddy RM, Gorrepati ML, Oh DS, et al. Robotic-Assisted Versus Thoracoscopic Lobectomy Outcomes From High-Volume Thoracic Surgeons. Ann Thorac Surg 2018;106:902-8. [Crossref] [PubMed]
- Di Lorenzo N, Coscarella G, Faraci L, et al. Robotic systems and surgical education. JSLS 2005;9:3-12. [PubMed]
- Narazaki K, Oleynikov D, Stergiou N. Robotic surgery training and performance: identifying objective variables for quantifying the extent of proficiency. Surg Endosc 2006;20:96-103. [Crossref] [PubMed]
- Jang HJ, Lee HS, Park SY, et al. Comparison of the early robot-assisted lobectomy experience to video-assisted thoracic surgery lobectomy for lung cancer: a single-institution case series matching study. Innovations (Phila) 2011;6:305-10. [Crossref] [PubMed]
- Suda T. Transition from video-assisted thoracic surgery to robotic pulmonary surgery. J Vis Surg 2017;3:55. [Crossref] [PubMed]
- Bhora FY, Al-Ayoubi AM, Rehmani SS, et al. Robotically Assisted Thoracic Surgery: Proposed Guidelines for Privileging and Credentialing. Innovations (Phila) 2016;11:386-9. [Crossref] [PubMed]
- The Institute for Surgical Excellence. Available online: http://surgicalexcellence.org/. Accessed July 27 2018.
- Fundamentals of Robotic Surgery. Available online: http://frsurgery.org/. Accessed July 27 2018.
- American College of Surgeons/Association of Program Directors in Surgery Surgical Skills Curriculum for Residents 2010. Available online: https://www.facs.org/education/program/resident-skills. Accessed July 27 2018.
- Zevin B, Levy JS, Satava RM, et al. A consensus-based framework for design, validation, and implementation of simulation-based training curricula in surgery. J Am Coll Surg 2012;215:580-6.e3. [Crossref] [PubMed]
- Park BJ, Flores RM. Cost comparison of robotic, video-assisted thoracic surgery and thoracotomy approaches to pulmonary lobectomy. Thorac Surg Clin 2008;18:297-300. vii. [Crossref] [PubMed]
- Herron DM, Marohn M. A consensus document on robotic surgery. Surg Endosc 2008;22:313-25; discussion 311-2. [Crossref] [PubMed]
- Veronesi G, Dorn P, Dunning J, et al. Outcomes from the Delphi process of the Thoracic Robotic Curriculum Development Committee. Eur J Cardiothorac Surg 2018;53:1173-9. [Crossref] [PubMed]
- Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 2008;18:289-95. vi-vii. [Crossref] [PubMed]
- Wu WB, Xu XF, Wen W, et al. Three-dimensional computed tomography bronchography and angiography in the preoperative evaluation of thoracoscopic segmentectomy and subsegmentectomy. J Thorac Dis 2016;8:S710-5. [Crossref] [PubMed]
- Shimizu K, Nakazawa S, Nagashima T, et al. 3D-CT anatomy for VATS segmentectomy. J Vis Surg 2017;3:88. [Crossref] [PubMed]
Cite this article as: Wahl TS, Wei B. Surgical simulation in robotic-assisted thoracic surgery: training. Video-assist Thorac Surg 2018;3:46.


