VATS-lymph node dissection, staging and restaging in advanced malignancy/the Munich experience
Background and objectives
In contrast to most other cancers, prognosis and survival have not significantly improved in lung cancer within the last decades. Despite evolving knowledge of lung cancer’s molecular genetics, improved ways of detection and staging, the overall 5-year survival has remained below 20% and is thus still very poor (1). While targeted and immunotherapy are generating hope for subgroups of patients with specific mutations in advanced stages (2,3), it is not the tumor’s molecular status but the exact clinical staging which determines therapy in early stages. In particular, oncologists and thoracic surgeons focus on the hilar and mediastinal lymph nodes since they determine prognosis and therapy. Of the three components of the TNM staging system, the most difficult one to determine clinically is the nodal component. The reference standard for staging this component is systematic lymph node dissection during anatomic tumor resection. Only an accurate staging can prevent futile surgery or identify those patients who benefit from induction therapy (4). There are several studies showing a significant survival benefit in patients with N2 disease if surgery is performed after induction therapy and the mediastinum was cleared of tumor (5-8). Therefore, an accurate primary staging (pre-induction) and restaging (post-induction) of the mediastinum is of utmost importance in NSCLC (9).
In the last two decades the introduction of integrated positron emission tomography and computed tomography (PET/CT) has helped to improve preinvasive mediastinal staging significantly (10). However, the limited specificity of imaging techniques necessitates invasive staging for histologic confirmation. The development of endoscopic ultrasound-guided fine needle aspiration (EBUS-TBNA) represents a significant clinical advancement, which has replaced mediastinoscopy—the traditional preresection staging method—nearly completely as the primary staging method. However, the high false-negative rate of bronchoscopic transbronchial fine needle aspiration (TBNA) with or without EBUS, indicates more invasive surgical staging when such testing is negative (11). Also, endoscopic restaging results after neoadjuvant therapy are not satisfactory (12). Therefore, despite these advances in imaging and endoscopic techniques, surgical staging still has a role in the management of lung cancer. In the last years, transcervical extended mediastinal lymphadenectomy (TEMLA) and video-assisted mediastinal lymphadenectomy (VAMLA) both modifications of classic mediastinoscopy have been developed to enable systematic lymph node dissection instead of sampling.
The objective of the following review is to give an overview on current standards of mediastinal staging for NSCLC followed by the presentation of the authors’ institutional practice.
Primary mediastinal lymph node staging
Imaging studies
CT-scanning of the chest
Computed tomography (CT) of the chest is part of the traditional work-up and the most widely available and most commonly used modality for evaluation of the mediastinum in lung cancer. Especially IV contrast CT provides very detailed anatomical information as well as morphologic information of the tumor, but has limited usefulness in the assessment of the mediastinal N-component. Generally, a larger diameter than 1 cm in the short axis is considered as the standard criterion for a suspicious lymph node. However, metastases of small nodes have been found in up to 20% in patients with clinical stage cT1-3N0, and only about 50% of the nodes with a diameter of 1.5 to 2 cm are metastasis. Therefore, lymph-node size is not a good predictor for malignancy. CT showed a low sensitivity of 55% and specificity of 81% in several meta-analyses (11,13). This performance is inadequate for therapeutic decisions, but sometimes CT can be useful in selecting the most appropriate procedure for tissue sampling. CT scanning of the thorax is clearly an imperfect means of staging the mediastinum, but it remains the best overall anatomic study available for the thorax and should be routinely performed in all patients with lung cancer.
PET scan and PET-CT
The introduction of integrated positron emission tomography (PET) with 18F-fluoro-2-deoxy-D-glucose with CT has improved non-invasive staging of lung cancer substantially. PET provides additional information about the metabolism of the cells. The main disadvantage of PET is its poor anatomical resolution. The exact localization of a single focal abnormality is almost impossible by means of PET alone. Around the turn of the millennium, integrated PET-CT scanners were introduced. The great advantage of this technology is the precise anatomical correlation of the radionuclide uptake.
Several meta-analyses have demonstrated that PET-CT is superior to CT for mediastinal staging in potential operable non-small-cell lung cancer. The overall sensitivity of PET-CT was 80–90% and the specificity was 85–95% (14-18). In comparison to mediastinoscopy, similar sensitivities and negative predictive values have been observed for PET. However, because inflammatory processes also take up FDG, the positive predictive value and specificity of FDG-PET are lower than in mediastinoscopy. PET-CT has a very high negative predictive value (94%) for detecting mediastinal nodal disease in peripherally located NSCLC. Due to this fact, the current North American and European guidelines suggest that in these patients further invasive staging procedures can be omitted and patients can be referred directly to surgery (11,13). However, if there is suspicion of N1-disease, the tumor is larger than 3 cm or is located centrally without suspected nodes on CT or PET/CT further invasive staging is recommended. In case of positive mediastinal PET, invasive mediastinal staging is still needed to confirm lymph-node metastasis.
Diffusion-weighted magnetic resonance imaging
Diffusion weighted imaging (DWI), an MRI technique, detects the restricted diffusion of water molecules among tissues at the cellular level. DWI has been widely used in brain imaging for the evaluation of acute ischemic stroke, intracranial tumors and demyelinating disease (19,20). However, DWI is highly sensitive to motion artifacts caused by breathing and movement of the heart, limiting it application (21). Recently, the rapid development of MRI techniques has allowed the application of DWI in areas prone to motion artifacts, such as the mediastinum (22). In a recent meta-analysis the diagnostic accuracy for DWI and PET/CT were analyzed (23). The pooled sensitivity for DWI was 0.72 (0.68–0.76) and specificity 0.97 (0.96–0.98). In comparison PET/CT achieved a pooled sensitivity and specificity of 0.65 (0.63–0.67) and 0.93 (0.93–0.94), respectively. The comparison of both modalities showed no significant differences in the diagnostic capacity of detecting mediastinal lymph nodes metastases in lung cancer. However, at the moment the true value of DWI remains unknown, as large-scale, prospective studies are needed to further justify the diagnostic value of DWI in comparison with PET/CT in clinical practice. In the future DWI might be an alternative modality for evaluating nodal status of NSCLC.
Endoscopic techniques
Endobronchial ultrasound for mediastinal lymph node staging
Until just about 20 years ago, endoscopic mediastinal lymph node staging was performed as transbronchial needle aspiration (TBNA)during bronchoscopy. This technique has a moderate yield (station 4,7), is a “blind” technique, is operator dependent, and the results also depend on the size of the lymph node. A sensitivity of 78% and a false negative rate of 28% were reported for conventional TBNA in clinical N2 disease (24). In the early 1990’s radial probe endobronchial ultrasound was introduced, which enabled for the first time to guide TBNA in patients with suspicion of mediastinal involvement in NSCLC. But it was not until the early 2000’s and introduction of convex probe EBUS (CP-EBUS) that mediastinal LN staging in lung cancer has undergone a revolutionary change. Up until recently, mediastinoscopy was the gold standard for mediastinal lymph node staging. However, EBUS-TBNA has shown to be equivalent to mediastinoscopy (25) and has demonstrated even better results in some studies (26,27). CP-EBUS is a flexible bronchoscope with a convex ultrasound transducer at the tip, which scans parallel to the insertion direction of the bronchoscope. This allows real time TBNA of the visualized structures. The current generation of ultrasound probes has B-mode and power color Doppler capabilities, allowing identification of vascular structures. The yield of EBUS-TBNA is comparable with mediastinoscopy (station 2R, 2L, 4R, 4L, 7) but in addition the hilar (station 10) and intrapulmonary (station 11) nodal stations can be approached. Posteriorly and deep located station 7 lymph nodes can be more easily assessed with EBUS-TBNA than with traditional mediastinoscopy (26,27). EBUS-TBNA is a safe procedure which can be performed in an outpatient setting (28). Several meta-analysis and systematic reviews of EBUS-TBNA reported an excellent overall sensitivity and specificity for mediastinal staging in NSCLC of 88–93% (95% CI, 0.79–0.94) and 1.00 (95% CI, 0.92–1.00), respectively. NPV of 91% (13,25,29-31).
Recently a new ultrasound processor has been developed, allowing real time assessment of lymph node deformability, which may be altered by tumor involvement. This so called elastography function takes advantage of the tissue distortion caused by vibrations of the heartbeat and vascular pulsations and might improve diagnostic performance (32). The conventional CP-EBUS has also been refined further. TCP-EBUS is a newly introduced bronchoscope with a thinner tip and a better angulation than conventional CP-EBUS. This allows also the assessment of the segmental bronchus and a better possible access of N1-stations. Further studies are needed for evaluation of these new technologies.
Esophageal ultrasound and combined ultrasonography for mediastinal lymph node staging
The assessment of the mediastinum has also been evaluated with esophageal ultrasound fine needle aspiration (EUS-FNA). EUS-FNA can be used complimentary to EBUS. It can particularly visualize stations 4L, 7, 8 and 9. It also offers access to the left adrenal gland. A meta-analysis showed a pooled sensitivity of 89%, a specificity of 100%, a positive predictive value of 100% and a negative predictive value of 86% (13). The combined ultrasonography (CUS) adds EUS-FNA to EBUS-TBNA enabling access of the mediastinal stations 2R, 2L, 4R, 4L, [5], 7, 8, and 9. CUS has shown to improve the access to the mediastinum (33) and to improve the diagnostic yield as compared to EBUS alone (25). A systematic review showed that the combined technique is more sensitive than EBUS-TBNA or EUS-FNA alone. In a recent meta-analysis based on eight studies CUS showed a pooled sensitivity of 86% (95% CI, 82–90%) with a 100% specifity (34). Also up to date no RCTs have been performed comparing CUS versus either EBUS-TBNA or EUS-FNA alone. The current ESTS guideline on preoperative mediastinal staging has integrated the possibility of CUS into their algorithm (11,35).
Surgical techniques
Mediastinoscopy
Carlens introduced cervical mediastinoscopy in 1959. It is a surgical biopsy technique usually performed under general anesthesia. The procedure involves a pretracheal suprasternal incision, insertion of a mediastinoscope anterior to the trachea, and biopsy of mediastinal nodes. For many years it has been the gold standard for invasive mediastinal staging of patients with lung cancer. Rates of morbidity and mortality are low (2% and 0.08%). Via this approach, right and left, high and low paratracheal nodes (stations 2R, 2L, 4R, 4L), pretracheal nodes (stations 1, 3), and anterior subcarinal nodes (station 7) are accessible (11,13). The median sensitivity of standard cervical mediastinoscopy is 78% with an NPV of 91% (13).
In 1995 the introduction of the video mediastinoscope lead to video assisted mediastinoscopy (VAM). VAM allows for better visualization and thus more extensive sampling (including posterior station 7). The median sensitivity of VAM to detect mediastinal node involvement from lung cancer was 89% in 995 patients. The median NPV was 92% (13).
There is no international standard of how many lymph nodes should be sampled during mediastinoscopy, but it has been suggested that ideally five nodal stations (stations 2R, 4R, 7, 4L, and 2L) should be examined routinely, with at least one node sampled from each station (36). The technique of mediastinoscopy seems relatively simple to perform, but should be reserved for experienced centers due to the danger of injuring the left recurrent nerve and some large vessels (superior vena cava, right pulmonary artery, innominate vein and brachio-cephalic trunk). However the morbidity and mortality of this staging technique are very low in experienced hands (9).
Video-assisted mediastinal lymphadenectomy
VAMLA is a further development of VAM, which enables complete and en-bloc removal of all mediastinal nodes and mediastinal fatty tissue that are accessible by VAM. The method was pioneered by Hürtgen and colleagues (37) at the beginning of the 2000s. It requires the same anesthetic considerations and patient positioning used for standard mediastinoscopy: general anesthesia and hyperextension of the neck with the patient in the supine decubitus position. A spreadable video mediastinoscope is used to enlarge the operative field, allowing better exposure of the mediastinal structures as well as bimanual dissection. The same instruments can be used as in standard mediastinoscopy: dissection-suction-coagulation cannula, grasping forceps, endoscopic scissors, biopsy forceps, and endoscopic clipping devices. Coagulation and cutting based on ultrasonography or with electrothermal tissue sealing devices are helpful to avoid left recurrent laryngeal nerve injury (38).
For the proposed VAMLA dissection technique the mediastinum is divided into three compartments: central, right, and left. The central compartment incudes the subcarinal (station 7) and the upper part of the paraesophageal nodes (station 8). Station 7 is considered to be completely dissected if 3 cm of each main bronchus are freed. All tissue below is defined as station 8. Dissection of station 8 is limited due to the accessibility of the mediastinoscope. The right compartment is composed of the pretracheal, right paratracheal, and right tracheobronchial nodes (stations 2R, 3 and 4R). The left compartment consists of the left paratracheal and tracheobronchial nodes (stations 4L and 2L). From the central and right compartment, the adipose tissue containing lymph nodes is excised en bloc along the anatomical landmarks of the mediastinum. Finally the left compartment is carefully dissected without the use of power devices (39).
Several studies showed a very good diagnostic accuracy of VAMLA, as lymphadenectomy is performed instead of nodal biopsy. Sensitivity was between 88–100% and NPV was 98–100% (37,38,40,41). But it also has been shown to have a higher complication rate with a morbidity rate ranging from 6.25% to 9%. The most common complication is recurrent laryngeal nerve palsy, occurring in 3.4% to 9% of patients after VAMLA (41,42). As the procedure is only performed in a few very experienced centers and evidence is still limited, VAMLA has not been recommended in the revised ESTS guideline as a standard tool for preoperative staging in patients with NSCLC.
In 2006, Witte et al. published their pooled experience with 144 VAMLA procedures performed over a 5-year period (41). The mean operative time was 54.1 min. In 13.2% the procedure was considered incomplete. The reasons for this included calcified lymph nodes, scarring, cervical spine deformities, extreme obesity, extra-nodal tumor growth or intra-operative complications. The authors emphasized that, in some cases, it is wiser to leave some lymph nodes and avoid risky dissection of vital structures. The authors noted that, with increasing experience, the rate of complications dropped from 5.3% in the first half of procedures to 2.6% in the second half.
A recent prospective observational study by Call et al. evaluated the results of VAMLA for staging of NSCLC in 185 consecutive patients. The staging protocol was based on the ESTS guidelines, but VAMLA was indicated instead of EBUS-FNA/EUS-FNA or mediastinoscopy to explore the mediastinum in the following situations: Central tumors, cN1 on CT or PET, tumors >3 cm, left-sided tumors, bilateral synchronous tumors, complex operations and in elderly patients or patients with poor performance status. VAMLA was contraindicated in patients with extensive mediastinal disease, for whom EBUS-FNA/EUS-FNA or mediastinoscopy may be enough to confirm nodal disease.
The rate of unsuspected N2–3 disease in the whole series was 18% (26 cases identified following VAMLA and 3 cases following tumor resection). The rate of unsuspected N2–3 disease depending on the presurgical cTNM status was 40.7% for cN1 tumors, 22.2% for cN0 tumors and tumor size equal to or greater than 3 cm, and 6.4% for cN0 tumors and tumor size less than 3 cm. The authors concluded that VAMLA should be included into the current staging algorithms, as the rate of unsuspected mediastinal nodal disease detected by VAMLA in cN1 tumors and cN0 tumors with tumor size larger than 3 cm was high.
Transcervical extended mediastinal lymphadenectomy
The transcervical extended mediastinal lymphadenectomy is an extension of traditional cervical mediastinoscopy using a 5–6 cm collar incision, sternal elevation and VAM. The published data for this technique are mainly from a highly experienced center in Zakopane, Poland. The operation begins with a cervical collar incision above the sternal notch. A sternal lift is performed with a retraction system to elevate the manubrium and increase the diameter of the thoracic outlet. The dissection starts at the right side in a plane over the brachiocephalic artery so that the right paratracheal space can be dissected out by direct visualization. The next step is establishing a pretracheal plane of dissection to dissect out the subcarinal space. The mediastinoscope is used for dissection of station 7 and 8 only. Then the left paratracheal space is dissected, sparing the left recurrent laryngeal nerve. Finally, the aortopulmonary lymph nodes are dissected after mobilization of the brachiocephalic vein. Using TEMLA stations 1, 2L, 4L, 3A, 2R, 4R, 7, 8, 5, and 6 can be dissected (4,43,44).
In 2011, Zieliński published a large study of a series of 698 TEMLA procedures. Sensitivity and negative predictive value of TEMLA for staging were 96.2% and 98.7%, respectively. The complication rate was reported to be 6.6%, and the mortality was 0.7%. The most frequent complication was recurrent laryngeal nerve injury (2.4% temporary nerve palsy, 0.3% permanent nerve palsy (45).
Video-assisted thoracoscopy (VATS)
VATS allows for access to almost every mediastinal lymph node station. It is performed under general anesthesia and usually requires double lumen intubation and, in general, it can only evaluate one side of the mediastinum.
On the right side all nodal stations are reached via a VATS approach. On the left side, however, access to the paratracheal nodes is more difficult and thus not routinely performed by the majority of VATS surgeons. No mortality has been reported from VATS mediastinal staging, and complications were noted in only 12 of 669 patients (average, 2%; range, 0–9%) (13). VATS is particularly indicated for biopsy of suspicious lymph nodes at station 5 and 6 (11), as large tissue samples can be obtained and these lymph node stations cannot be biopsied by routine mediastinoscopy and EBUS-TBNA. The performance characteristics of VATS mediastinal node biopsy for N2 node staging are quite good with a sensitivity of 99% and a NPV of 96% (13).
Aorto-pulmonary window
An alternative to VATS for accessing station 5 and 6 is left anterior mediastinotomy (Chamberlain procedure). In some experienced centres, also extended mediastinoscopy from the mediastinoscopy incision is performed for these lymph node stations with very good results (NPV: 0.89–0.97).
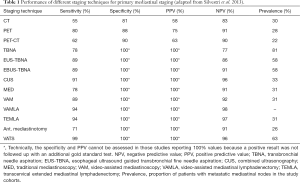
An overview of the performance of different staging techniques for primary mediastinal staging is given in the following Table 1.
Full table
Restaging
About 15% of the patients diagnosed with NSCLC are in stage IIIA (N2) (46). In many institutions it has become a common practice to consider a multimodal therapeutic approach for these patients with preoperative induction therapy (chemotherapy ± radiotherapy) followed by possible surgery. There is no consensus as to the optimal management of his stage, nor for the role of surgery. It has been shown if induction therapy achieves clearance of the mediastinal nodes (downstaging) it is a major prognostic factor (6,8,47). If surgical resection adds further benefit over chemoradiation and if surgery should only be offered to patients with clearance of the mediastinal nodes is controversial (48). However, it has to be considered, that resection following induction therapy increases the postoperative morbidity and mortality compared with resection without induction treatment (49). Therefore if surgery is depending on a proper mediastinal downstaging, it is important to know which restaging test can accurately predict the mediastinal downstaging.
Imaging
CT-scanning was shown to have a low accuracy in primary mediastinal staging, and in mediastinal restaging it is even lower. Only few studies have formally evaluated the reliability of restaging by CT (47,50-54). In a systematic review about mediastinal restaging a pooled sensitivity of 63%, a specificity of 70%, a false negative rate of 31% and false positive rate of 34% were reported (55).
PET-scan alone for re-staging is less sensitive than before induction therapy. PET has been shown to be more accurate in predicting the T component than the N status (56). The average sensitivity, specificity, false negative rate and false positive rate across nine studies (50,53,57-63) were 60%, 66%, 27% and 38%, respectively (55).
Compared to the PET-scan alone, the integrated PET-CT significantly increases the specificity through better localization of focal FDG uptake within the mediastinum (50). Change in SUVmax values of PET/CT scans before and after induction therapy allow for prediction of the histopathological response in the primary tumor and mediastinal lymph nodes and thus has a prognostic value (64). Several studies have shown that high residual activity in the primary tumor and in mediastinal nodes is associated with a poor prognosis. An average sensitivity of 70%, specificity of 90%, NPV 23% and PPV 16% were reported for PET-CT as a result of two studies on this topic (50,55,65). In a prospective study by Cerfolio et al. of 93 patients who were restaged by chest CT and integrated PET–CT after induction therapy, PET–CT was found to be more accurate than CT alone. But as there were 20% false-negative and 25% false-positive cases, nodal biopsies are still required, in case of suspicion of residual mediastinal disease (65).
Endoscopy
Endoscopic techniques are an alternative to re-mediastinoscopy for biopsies and thus histologic proof in restaging; EBUS and EUS after induction therapy are less technically challenging and associated with lower morbidity. Candela and Detterbeck reported of an overall sensitivity and false negative rate for mediastinal restaging with endoscopic needle techniques (EBUS-TBNA and CUS) of 63% and 14%, respectively. These results suggest that negative results for restaging obtained by endoscopy should be confirmed by invasive surgical mediastinal restaging.
VAM
Remediastinoscopy is technically feasible after induction therapy (66,67). This technique can often be challenging, due to fibrosis and adhesions. Only a small number of centers have reported their experience with repeat mediastinoscopy. The pooled sensitivity and false negative rate to detect residual mediastinal disease were 63% and 22%, respectively. The average feasibility of remediastinoscopy was 87% (50,55,66,68-70). Overall the studies showed a lower accuracy in comparison with primary mediastinoscopy (11).
Lardinois et al. reported a sensitivity of 82% and a FN rate of 15% for first time mediastinoscopy for mediastinal restaging after primary endoscopic staging.
VAMLA
There are no published data about the use of VAMLA in mediastinal restaging.
TEMLA
TEMLA delivered very accurate results in restaging after neoadjuvant therapy. Zielinski et al. published in 2010 a study in which 63 patients with N2/N3-NSCLC were restaged after induction therapy with TEMLA. Sensitivity, specificity, NPV and accuracy of TEMLA were 95.7%, 100%, 97.6% and 98.4%, respectively (71). In 2013 the same author published a retrospective analysis comparing EBUS/EUS and TEMLA performed for restaging after neoadjuvant treatment. Sensitivity, specificity and NPV of TEMLA were 96.2%, 100% and 99.6%, respectively (72).
These results are better than any other method of restaging short of surgical resection but since the technique is associated with a relatively high morbidity and is only available in few centers, the currents guidelines on preoperative mediastinal staging do not recommend TEMLA as routine diagnostic outside of clinical trials (73).
VATS
There is only one study about the feasibility of VATS for mediastinal restaging after induction therapy. In this Cancer and Leukemia Group B 39803 prospective multi-institutional trial a total of 47 VATS procedures were performed between 1998 and 2003; sensitivity, specificity and NPV of VATS were 67%, 100% and 73%, respectively; a negative result was defined if negative lymph node biopsies from at least three lymph node stations could be obtained, whereas a positive result consisted of a proof of persisting N2 disease or the demonstration of pleural carcinomatosis. The VATS approach was not feasible in 21 patients (31%).
An overview of the performance of different staging techniques for mediastinal restaging after induction therapy is shown in Table 2.

Full table
Clinical mediastinal lymph-node staging in our institution
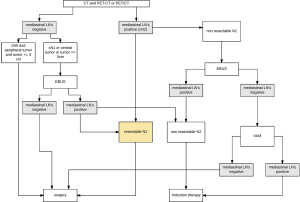
In accordance to the ongoing rethinking of the impact of mediastinal staging and induction therapy in NSCLC (“Invasive mediastinal staging is irrelevant for PET/CT positive N2 lung cancer if the primary tumor and ipsilateral lymph nodes are resectable”) (74) we recently have modified our institutional algorithm in respect to the available staging techniques the following way (Figure 1):
- Direct surgery is recommended if all of these three criteria apply: cN0 on CT or PET, tumor size ≤3 cm (stage IA), tumor located in the outer third of the lung.
- In case of enlarged mediastinal lymph nodes on CT and/or PET-positive lymph nodes tissue confirmation is sought if surgical respectability of the specific N2 lymph nodes is questionable. In this case, endosonography is our first choice. If negative, VAM is performed. For patients with a left upper lobe tumor, surgical staging of the aorto-pulmonary window nodes (if enlarged on CT and/or PET-CT-positive) is performed by VATS if involvement changes treatment strategy.
- Invasive staging by EBUS is performed if at least one of these criteria applies: central lesion, suspect N1 nodes, tumor size >3 cm. If negative and N2 is considered resectable, surgery is performed. If negative and N2 would not be considered as resectable, VAM is performed. If VAM is negative, these patients undergo surgical treatment. They also undergo surgical treatment after negative EBUS if the number of nodes explored and the number of needle passes in each node meet the established requirements. Otherwise, surgical exploration is recommended after negative EBUS.
The definition of what is meant by “resectable”, “marginally resectable”, and “unresectable” is not clearly determined but highly subjective and dependent on the experience and expertise of the thoracic surgeon. In our institution we use the proposed subset classification by the IASLC of single level N2 (N2a) and multilevel N2 (N2b) (75) as well as subset classification of Stage IIIA by Robinson. Single level stage IIIA3 (N2a) is regarded to be resectable (76) (see Table 3).
Summary restaging
We regard mediastinal restaging after induction therapy as necessary to select those patients who most likely will benefit from surgery. As in our setting most of the patients with stage IIIA1-3 undergo primary surgery and only patients with initially not resectable N2 (IIIA4) disease are referred to neoadjuvant therapy, we rarely have good candidates for surgical restaging that might profit from adjuvant surgery.
It is not possible to rely on imaging techniques only, as they do not accurately determine the tumor’s biological response to induction therapy. An invasive technique providing histological information is thus necessary. Depending on the clinical situation we use EBUS, VATS of VAM. If N3 is suspected after induction therapy EBUS and if negative followed by 1st time VAM is our first choice.
Discussion
The optimal management of clinical N2 Stage IIIA non-small cell lung cancer is still controversial. There is a substantial mismatch between the quite high evidence and the little consensus of how to handle this subgroup of patients with resectable primary disease in the presence of non-fixed, non-bulky N2 metastases (74).
As concluded in a review by Bakir et al. we believe that surgery, as part of a multimodal therapeutic approach, offers a survival benefit for patients with resectable N2 NSCLC. Overall 5-year survival rates following primary resection ranged from 17% to 20% (four studies). Improved 5-year survival was demonstrated with multimodality therapy (19–45%; 13 studies) (77).
Historically, stage IIIA/N2 lung cancer has been referred to primary surgery up until the 1990s (78). The rather poor oncologic outcome of patients in this group and the high morbidity and mortality associated with open thoracic surgery raised questions if surgery was really indicated for this subgroup—at all or as the fist therapeutic step (79,80). It is important to emphasize that a systematic lymph node dissection was not a routine part of an anatomical lung resection at that time (81). There are no randomized controlled studies that compare adjuvant chemotherapy vs. “adjuvant” surgery in a multimodal therapeutic concept for stage IIIA/N2 lung cancer, especially in cases of resectable N2-disease.
VATS lung resection with systematic mediastinal lymph node dissection has proven to have less mortality and morbidity than lung resection via an open approach (82). In experienced institutions a standardized technique of systematic mediastinal lymph node dissection by VATS can provide very accurate results with complete en-bloc resection of all relevant lymph node stations (83,84). Even though a recent review by Zhang et al comparing accuracy of lymph node dissection between a conventional open (thoracotomy) vs. a thoracoscopic approach in pulmonary lobectomy has suggested a superiority of thoracotomy, it has been shown that VATS enables for the same results with regard to the number and weight of lymph nodes dissected (85,86).
A retrospective analysis of the U. S. National Cancer Database comparing patients with clinical stage N0 and N2 who after surgery all were found to be pathologically N2 and thus underwent adjuvant chemotherapy showed no significant difference in 5-year overall survival (40% vs. 37%, P=0.167, n=3,271) (87). This was confirmed by Tsitsias et al. who did not find significant survival differences between clinical (based on preoperative PET-CT) N0 patients who nevertheless were found to have pN2 disease on resection and those who were already single-zone cN2 positive on preoperative clinical staging (88).
We therefore believe and follow the strategy that a—VATS!—anatomic tumor resection with systematic lymph node dissection is justified as the primary therapeutic step in patients with resectable stage IIIA lung cancer; its low morbidity does not impair or postpone the following parts of an—in any case!—indicated multimodality treatment regimen. Randomized prospective studies are needed to prove this concept.
If following this logic, also the algorithm for this subgroup of patients who receive neoadjuvant therapy has to be revised, as only patients with multi-level N2 (N2b) and/or bulky N2 (IIIA4) remain potential candidates. For restaging after induction therapy, PET/CT was shown to have a high FN rate of about 23% and a FP rate of 16% for all types of N2 disease (55). There are no studies evaluating the accuracy of different restaging techniques in the subgroup of N2A- and/or IIIA4 patients. However, given the high FN rate of PET/CT and the poor survival in this group if downstaging is not achieved, invasive restaging seems to be mandatory.
The role of VAM and VATS for restaging has not been generally set yet. There is only one study by Jaklitsch et al. addressing VATS as a restaging technique (89). The study enrolled 75 patients between 1998 and 2003. Sensitivity, specificity and NPV of VATS for restaging were 67%, 100% and 73%, respectively. The authors reported a feasibility of 40%. Due to obliteration of some nodal stations after neoadjuvant therapy, complete staging of a least 3 nodal stations was not possible in all patients, so that the pre-study definition of success was not achieved. 29% of the evaluated patients had at least 1 nodal station staged by VATS with no remaining nodal tissue and were confirmed to be pN0 on thoracotomy with nodal dissection. By including these patients as successfully restaged into the calculation, the estimated feasibility would be 69%. According to the authors, these results do not justify to establish VATS as a restaging technique. Considering the increasing experience in standardized lymph node dissection gained in routine VATS-lobectomies within the last decade, its current feasibility and diagnostic accuracy is expected to be much higher and may need to be reevaluated, however.
If feasible and safe, VATS is an ideal tool to definitively clear histology once PET/CT and/or EBUS/EUS-TBNA suggest complete response to induction therapy: if the frozen section of the completely dissected mediastinal lymph nodes, that were initially proven to be positive by biopsy do not show any remnant tumor invasion, proceeding with VATS surgical resection of the primary tumor may be the best treatment option.
In the following videos 1-5 our dissection technique is demonstrated for the relevant anatomical regions in NSCLC (video 1: paratracheal lymph nodes on the right side; video 2: subcarinal lymph nodes from the right side; video 3: subcarinal lymph nodes from the left side; video 4: inferior pulmonary ligament on the right side; video 5: lymph nodes in the aorto-pulmonary window
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Todd Demmy) for the series “VATS for Locally Advanced Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.06.02). The series “VATS for Locally Advanced Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Malhotra J, Jabbour SK, Aisner J. Current state of immunotherapy for non-small cell lung cancer. Transl Lung Cancer Res 2017;6:196-211. [Crossref] [PubMed]
- Revannasiddaiah S, Thakur P, Bhardwaj B, et al. Pulmonary adenocarcinoma: implications of the recent advances in molecular biology, treatment and the IASLC/ATS/ERS classification. J Thorac Dis 2014;6:S502-25. [PubMed]
- Yendamuri S, Demmy TL. Is VAMLA/TEMLA the new standard of preresection staging of non-small cell lung cancer? J Thorac Cardiovasc Surg 2012;144:S14-7. [Crossref] [PubMed]
- Betticher DC, Hsu Schmitz SF, Tötsch M, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol 2003;21:1752-9. [Crossref] [PubMed]
- Bueno R, Richards WG, Swanson SJ, et al. Nodal stage after induction therapy for stage IIIA lung cancer determines patient survival. Ann Thorac Surg 2000;70:1826-31. [Crossref] [PubMed]
- Lorent N, De Leyn P, Lievens Y, et al. Long-term survival of surgically staged IIIA-N2 non-small-cell lung cancer treated with surgical combined modality approach: analysis of a 7-year prospective experience. Ann Oncol 2004;15:1645-53. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Lardinois D. Pre- and intra-operative mediastinal staging in non-small-cell lung cancer. Swiss Med Wkly 2011;141:w13168 [PubMed]
- Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med 2003;348:2500-7. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Preoperative mediastinal lymph node staging for non-small cell lung cancer: 2014 update of the 2007 ESTS guidelines. Transl Lung Cancer Res 2014;3:225-33. [PubMed]
- Nasir BS, Bryant AS, Minnich DJ, et al. The efficacy of restaging endobronchial ultrasound in patients with non-small cell lung cancer after preoperative therapy. Ann Thorac Surg 2014;98:1008-12. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest 2003;123:137S-46S. [Crossref] [PubMed]
- Dwamena BA, Sonnad SS, Angobaldo JO, et al. Metastases from non-small cell lung cancer: mediastinal staging in the 1990s--meta-analytic comparison of PET and CT. Radiology 1999;213:530-6. [Crossref] [PubMed]
- Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med 2003;139:879-92. [Crossref] [PubMed]
- Fischer BM, Mortensen J, Højgaard L. Positron emission tomography in the diagnosis and staging of lung cancer: a systematic, quantitative review. Lancet Oncol 2001;2:659-66. [Crossref] [PubMed]
- Birim O, Kappetein AP, Stijnen T, et al. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg 2005;79:375-82. [Crossref] [PubMed]
- Matoba M, Tonami H, Kondou T, et al. Lung Carcinoma: Diffusion-weighted MR Imaging—Preliminary Evaluation with Apparent Diffusion Coefficient. Radiology 2007;243:570-7. [Crossref] [PubMed]
- Usuda K, Sagawa M, Motono N, et al. Advantages of Diffusion-Weighted Imaging Over Positron Emission Tomography-Computed Tomography in Assessment of Hilar and Mediastinal Lymph Node in Lung Cancer. Ann Surg Oncol 2013;20:1676-83. [Crossref] [PubMed]
- Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 2007;188:1622-35. [Crossref] [PubMed]
- Pauls S, Schmidt SA, Juchems MS, et al. Diffusion-weighted MR imaging in comparison to integrated [18F]-FDG PET/CT for N-staging in patients with lung cancer. Eur J Radiol 2012;81:178-82. [Crossref] [PubMed]
- Shen G, Lan Y, Zhang K, et al. Comparison of 18F-FDG PET/CT and DWI for detection of mediastinal nodal metastasis in non-small cell lung cancer: A meta-analysis. PLoS One 2017;12:e0173104 [Crossref] [PubMed]
- Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S-20S.
- Czarnecka-Kujawa K, Yasufuku K. The role of endobronchial ultrasound versus mediastinoscopy for non-small cell lung cancer. J Thorac Dis 2017;9:S83-97. [Crossref] [PubMed]
- Um SW, Kim HK, Jung SH, et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J Thorac Oncol 2015;10:331-7. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]
- Yarmus LB, Akulian JA, Gilbert C, et al. Comparison of moderate versus deep sedation for endobronchial ultrasound transbronchial needle aspiration. Ann Am Thorac Soc 2013;10:121-6. [Crossref] [PubMed]
- Adams K, Shah PL, Edmonds L, et al. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009;64:757-62. [Crossref] [PubMed]
- Dong X, Qiu X, Liu Q, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the mediastinal staging of non-small cell lung cancer: a meta-analysis. Ann Thorac Surg 2013;96:1502-7. [Crossref] [PubMed]
- Sehgal IS, Dhooria S, Aggarwal AN, et al. Endosonography Versus Mediastinoscopy in Mediastinal Staging of Lung Cancer: Systematic Review and Meta-Analysis. Ann Thorac Surg 2016;102:1747-55. [Crossref] [PubMed]
- Gu Y, Shi H, Su C, et al. The role of endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Oncotarget. 2017;8:89194-202. [Crossref] [PubMed]
- Hwangbo B, Lee GK, Lee HS, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest 2010;138:795-802. [Crossref] [PubMed]
- Zhang R, Ying K, Shi L, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: a meta-analysis. Eur J Cancer 2013;49:1860-7. [Crossref] [PubMed]
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015;47:545-59. [Crossref] [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Hürtgen M, Friedel G, Toomes H, et al. Radical video-assisted mediastinoscopic lymphadenectomy (VAMLA)--technique and first results. Eur J Cardiothorac Surg 2002;21:348-51. [Crossref] [PubMed]
- Call S, Obiols C, Rami-Porta R, et al. Video-Assisted Mediastinoscopic Lymphadenectomy for Staging Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:1326-33. [Crossref] [PubMed]
- Witte B, Hürtgen M. Video-assisted mediastinoscopic lymphadenectomy. J Thorac Oncol 2007;2:367-9. [Crossref] [PubMed]
- Leschber G, Holinka G, Linder A. Video-assisted mediastinoscopic lymphadenectomy (VAMLA)--a method for systematic mediastinal lymphnode dissection. Eur J Cardiothorac Surg 2003;24:192-5. [Crossref] [PubMed]
- Witte B, Wolf M, Huertgen M, et al. Video-assisted mediastinoscopic surgery: clinical feasibility and accuracy of mediastinal lymph node staging. Ann Thorac Surg 2006;82:1821-7. [Crossref] [PubMed]
- Turna A, Demirkaya A, Ozkul S, et al. Video-assisted mediastinoscopic lymphadenectomy is associated with better survival than mediastinoscopy in patients with resected non-small cell lung cancer. J Thorac Cardiovasc Surg 2013;146:774-80. [Crossref] [PubMed]
- Kużdżał J, Szlubowski A, Grochowski Z, et al. Current evidence on transcervical mediastinal lymph nodes dissection. Eur J Cardiothorac Surg 2011;40:1470-3. [PubMed]
- Kuzdzał J, Zieliński M, Papla B, et al. Transcervical extended mediastinal lymphadenectomy--the new operative technique and early results in lung cancer staging. Eur J Cardiothorac Surg 2005;27:384-90; discussion 390. [Crossref] [PubMed]
- Zieliński M, Hauer L, Hauer J, et al. Transcervical Extended Mediastinal Lymphadenectomy (TEMLA) for staging of non-small-cell lung cancer (NSCLC). Pneumonol Alergol Pol 2011;79:196-206. [PubMed]
- Bülzebruck H, Bopp R, Drings P, et al. New aspects in the staging of lung cancer. Prospective validation of the International Union Against Cancer TNM classification. Cancer 1992;70:1102-10. [Crossref] [PubMed]
- Trodella L, Granone P, Valente S, et al. Neoadjuvant concurrent radiochemotherapy in locally advanced (IIIA-IIIB) non-small-cell lung cancer: long-term results according to downstaging. Ann Oncol 2004;15:389-98. [Crossref] [PubMed]
- Detterbeck F, Sukumar MS. Management Algorithms for Stage IIIA Non–Small Cell Lung Cancer with N2 Node Involvement. Thorac Surg Clin 2008;18:437-41. vii. [Crossref] [PubMed]
- Martin J, Ginsberg RJ, Venkatraman ES, et al. Long-Term Results of Combined-Modality Therapy in Resectable Non–Small-Cell Lung Cancer. J Clin Oncol 2002;20:1989-95. [Crossref] [PubMed]
- De Leyn P, Stroobants S, De Wever W, et al. Prospective Comparative Study of Integrated Positron Emission Tomography-Computed Tomography Scan Compared With Remediastinoscopy in the Assessment of Residual Mediastinal Lymph Node Disease After Induction Chemotherapy for Mediastinoscopy-Proven Stage IIIA-N2 Non–Small-Cell Lung Cancer: A Leuven Lung Cancer Group Study. J Clin Oncol 2006;24:3333-9. [Crossref] [PubMed]
- Mateu-Navarro M, Rami-Porta R, Bastus-Piulats R, et al. Remediastinoscopy after induction chemotherapy in non-small cell lung cancer. Ann Thorac Surg 2000;70:391-5. [Crossref] [PubMed]
- Lardinois D, Schallberger A, Betticher D, et al. Postinduction video-mediastinoscopy is as accurate and safe as video-mediastinoscopy in patients without pretreatment for potentially operable non-small cell lung cancer. Ann Thorac Surg 2003;75:1102-6. [Crossref] [PubMed]
- Ohtsuka T, Nomori H, Ebihara A, et al. FDG-PET imaging for lymph node staging and pathologic tumor response after neoadjuvant treatment of non-small cell lung cancer. Ann Thorac Cardiovasc Surg 2006;12:89-94. [PubMed]
- Cerfolio RJ, Ojha B, Mukherjee S, et al. Positron emission tomography scanning with 2-fluoro-2-deoxy-d-glucose as a predictor of response of neoadjuvant treatment for non-small cell carcinoma. J Thorac Cardiovasc Surg 2003;125:938-44. [Crossref] [PubMed]
- de Cabanyes Candela S, Detterbeck FC. A Systematic Review of Restaging After Induction Therapy for Stage IIIa Lung Cancer: Prediction of Pathologic Stage. J Thorac Oncol 2010;5:389-98. [Crossref] [PubMed]
- Dooms C, Vansteenkiste J. Prognostic value of fluorodeoxyglucose uptake in non-small cell lung cancer: time for standardization and validation. J Thorac Oncol 2010;5:583-4. [Crossref] [PubMed]
- Stigt JA, Oostdijk AH, Timmer PR, et al. Comparison of EUS-guided fine needle aspiration and integrated PET-CT in restaging after treatment for locally advanced non-small cell lung cancer. Lung Cancer 2009;66:198-204. [Crossref] [PubMed]
- Hoekstra CJ, Stroobants SG, Smit EF, et al. Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J Clin Oncol 2005;23:8362-70. [Crossref] [PubMed]
- Ryu JS1. FDG-PET in staging and restaging non-small cell lung cancer after neoadjuvant chemoradiotherapy: correlation with histopathology. Lung Cancer 2002;35:179-87. [Crossref] [PubMed]
- Eschmann SM, Friedel G, Paulsen F, et al. 18F-FDG PET for assessment of therapy response and preoperative re-evaluation after neoadjuvant radio-chemotherapy in stage III non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2007;34:463-71. [Crossref] [PubMed]
- Hellwig D, Graeter TP, Ukena D, et al. Value of F-18-fluorodeoxyglucose positron emission tomography after induction therapy of locally advanced bronchogenic carcinoma. J Thorac Cardiovasc Surg 2004;128:892-9. [Crossref] [PubMed]
- Akhurst T, Downey RJ, Ginsberg MS, et al. An initial experience with FDG-PET in the imaging of residual disease after induction therapy for lung cancer. Ann Thorac Surg 2002;73:259-64; discussion 264-6. [Crossref] [PubMed]
- Port JL, Kent MS, Korst RJ, et al. Positron emission tomography scanning poorly predicts response to preoperative chemotherapy in non-small cell lung cancer. Ann Thorac Surg 2004;77:254-9; discussion 259. [Crossref] [PubMed]
- Pöttgen C, Levegrün S, Theegarten D, et al. Value of 18F-fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in non-small-cell lung cancer for prediction of pathologic response and times to relapse after neoadjuvant chemoradiotherapy. Clin Cancer Res 2006;12:97-106. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Ojha B. Restaging patients with N2 (stage IIIa) non-small cell lung cancer after neoadjuvant chemoradiotherapy: a prospective study. J Thorac Cardiovasc Surg 2006;131:1229-35. [Crossref] [PubMed]
- Stamatis G, Fechner S, Hillejan L, et al. Repeat mediastinoscopy as a restaging procedure. Pneumologie 2005;59:862-6. [Crossref] [PubMed]
- Pauwels M, Van Schil P, De Backer W, et al. Repeat mediastinoscopy in the staging of lung cancer. Eur J Cardiothorac Surg 1998;14:271-3. [Crossref] [PubMed]
- Meersschaut D, Vermassen F, de laRivière AB, et al. Repeat mediastinoscopy in the assessment of new and recurrent lung neoplasm. Ann Thorac Surg 1992;53:120-2. [Crossref] [PubMed]
- Marra A, Hillejan L, Fechner S, et al. Remediastinoscopy in restaging of lung cancer after induction therapy. J Thorac Cardiovasc Surg 2008;135:843-9. [Crossref] [PubMed]
- De Waele M, Serra-Mitjans M, Hendriks J, et al. Accuracy and survival of repeat mediastinoscopy after induction therapy for non-small cell lung cancer in a combined series of 104 patients. Eur J Cardiothorac Surg 2008;33:824-8. [Crossref] [PubMed]
- Zieliński M, Hauer L, Hauer J, et al. Non-small-cell lung cancer restaging with transcervical extended mediastinal lymphadenectomy. Eur J Cardiothorac Surg 2010;37:776-80. [Crossref] [PubMed]
- Zielinski M, Szlubowski A, Kołodziej M, et al. Comparison of endobronchial ultrasound and/or endoesophageal ultrasound with transcervical extended mediastinal lymphadenectomy for staging and restaging of non-small-cell lung cancer. J Thorac Oncol 2013;8:630-6. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Lim E, McElnay PJ, Rocco G, et al. Invasive mediastinal staging is irrelevant for PET/CT positive N2 lung cancer if the primary tumour and ipsilateral lymph nodes are resectable. Lancet Respir Med 2015;3:e32-3. [Crossref] [PubMed]
- Rusch VW, Crowley J, Giroux DJ, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:603-12.
- Robinson LA, Ruckdeschel JC, Wagner H Jr, et al. Treatment of Non-small Cell Lung Cancer-Stage IIIA. Chest 2007;132:243S-65S. [Crossref] [PubMed]
- Bakir M, Fraser S, Routledge T, et al. Is surgery indicated in patients with stage IIIa lung cancer and mediastinal nodal involvement? Interact Cardiovasc Thorac Surg 2011;13:303-10. [Crossref] [PubMed]
- Mountain CF. The biological operability of stage III non-small cell lung cancer. Ann Thorac Surg 1985;40:60-4. [Crossref] [PubMed]
- Shields TW, Yee J, Conn JH, et al. Relationship of cell type and lymph node metastasis to survival after resection of bronchial carcinoma. Ann Thorac Surg 1975;20:501-10. [Crossref] [PubMed]
- Paulson DL, Reisch JS. Long-term survival after resection for bronchogenic carcinoma. Ann Surg 1976;184:324-32. [Crossref] [PubMed]
- Naruke T, Goya T, Tsuchiya R, et al. The Importance of Surgery to Non-Small Cell Carcinoma of Lung with Mediastinal Lymph Node Metastasis. Ann Thorac Surg 1988;46:603-10. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Amer K, Khan AZ, Singh N, et al. Video-assisted thoracic surgery systematic mediastinal nodal dissection and stage migration: impact on clinical pathway. Eur J Cardiothorac Surg 2011;40:1474-81. [PubMed]
- Reichert M, Steiner D, Kerber S, et al. A standardized technique of systematic mediastinal lymph node dissection by video-assisted thoracoscopic surgery (VATS) leads to a high rate of nodal upstaging in early-stage non-small cell lung cancer. Surg Endosc 2016;30:1119-25. [Crossref] [PubMed]
- Zhang W, Wei Y, Jiang H, et al. Thoracotomy is better than thoracoscopic lobectomy in the lymph node dissection of lung cancer: a systematic review and meta-analysis. World J Surg Oncol 2016;14:290. [Crossref] [PubMed]
- Palade E, Passlick B, Osei-Agyemang T, et al. Video-assisted vs open mediastinal lymphadenectomy for Stage I non-small-cell lung cancer: results of a prospective randomized trial. Eur J Cardiothorac Surg 2013;44:244-9; discussion 249. [Crossref] [PubMed]
- Thomas DC, Arnold BN, Rosen JE, et al. The Significance of Upfront Knowledge of N2 Disease in Non-small Cell Lung Cancer. World J Surg 2018;42:161-71. [Crossref] [PubMed]
- Tsitsias T, Boulemden A, Ang K, et al. The N2 paradox: similar outcomes of pre- and postoperatively identified single-zone N2a positive non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:882-7. [Crossref] [PubMed]
- Jaklitsch MT, Gu L, Demmy T, et al. Prospective phase II trial of preresection thoracoscopic mediastinal restaging after neoadjuvant therapy for IIIA (N2) non-small cell lung cancer: results of CALGB Protocol 39803. J Thorac Cardiovasc Surg 2013;146:9-16. [Crossref] [PubMed]
Cite this article as: Hiebinger A, Öfner-Velano D, Bodner J. VATS-lymph node dissection, staging and restaging in advanced malignancy/the Munich experience. Video-assist Thorac Surg 2018;3:29.