
Understanding the impact of neoadjuvant chemoradiation on health-related quality of life in esophageal cancer
In the recent manuscript “Effect of neoadjuvant chemoradiotherapy on health-related quality of life in esophageal or junctional cancer: results from the randomized CROSS trial”, Noordman et al. (1) compared health-related quality of life (HRQOL) outcomes between two groups of patients: those randomized to neoadjuvant chemoradiation (nCRT) followed by surgery and those treated with surgery alone for esophageal cancers. They evaluated five pre-specified quality of life measures in 363 patients who were participants in the CROSS trial, and compared the HRQOL of the groups at baseline and at three month intervals over the course of their first postoperative year. Their HRQOL outcomes included several measures: physical functioning, eating problems, global quality of life, fatigue, and emotional problems. They found no statistically significant difference between treatment groups in any measure at baseline, 3, 6, 9, and 12 months. All metrics declined in both groups at 3 months postoperatively, and improved thereafter. Physical functioning and fatigue remained below baseline at 12 months, while eating problems, global quality of life, and emotional problems returned to baseline by 6–9 months. The authors also evaluated patients undergoing nCRT 1 week after completion of their induction therapy, and found significant worsening of all measures at that time point compared to pre-treatment baseline, however this difference resolved by 3 months postoperatively. A detailed summary of their findings, including both absolute differences in mean scores and calculated standardized Cohen’s d effect sizes, is shown here in Table 1 for reference.
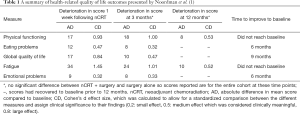
Full table
The CROSS trial (2) was a landmark randomized controlled trial that solidly established nCRT followed by esophagectomy as the standard of care for esophageal cancer because of the significant survival benefit observed in patients receiving nCRT. This trial included individuals with T1N1 or T2–3N0–1 esophageal cancer (75% adenocarcinoma, 23% squamous cell carcinoma). Patients were randomized to either surgery alone or trimodality therapy including: neoadjuvant chemotherapy with carboplatin and paclitaxel and 41.4 Gy of external beam radiation using a 3-D conformal radiation technique, followed by esophagectomy after a median interval of 6.6 weeks. The researchers found a significantly improved overall survival with median values of 49.4 months in the nCRT plus surgery group, compared to 24.0 months for the surgery alone group. The hazard ratio for death was 0.66 (95% CI: 0.50–0.87) favoring induction therapy, and these results were confirmed in long-term follow-up (3). This impressive survival advantage led to widespread adoption of the protocol, and the use of nCRT has focused concerns about the effect of nCRT on quality of life.
This current study on HRQOL by Noordman et al. is an important contribution since there is currently little high-quality literature on the subject. This was a well-planned study that rigorously evaluated HRQOL within a randomized clinical trial. The authors analyzed relevant metrics at specific time points throughout the first post-treatment year, seeking to better understand and document the patient experience of esophageal cancer treatment in a scientific manner. This granular data on patient-reported symptoms enriches the survival data from the original CROSS trial. It provides an important understanding of the symptomatic differences between treatment modalities: namely that patients can expect a transient worsening in all functional measures following nCRT, but they can also expect to return to the symptom profile otherwise expected after surgery alone by 3 months. This is not only very useful for counseling patients considering nCRT versus surgery alone, but also for informing patients on what to expect after an esophagectomy in general. All patients, regardless of treatment selection, can expect a deterioration in quality of life symptoms 3 months postoperatively that will subsequently improve. Physical functioning and fatigue may not quite return to baseline, but overall global quality of life, eating, and emotional symptoms will likely reach pretreatment levels.
There are several limitations of this study that should be considered. First, despite the advantages in scientific rigor that come with data from a randomized controlled trial, the inclusion criteria of the trial often select for patients healthier than those in the general population with the disease. The CROSS trial excluded patients older than 75 years of age, those with a limited performance status who were confined to a bed or chair >50% of the day (WHO three or greater), and those who had lost more than 10% of their body weight. Also excluded were those with prior cancer or a serious impairment of lung, liver, or kidney function. The excluded patients may be expected to be frailer than those who were eligible, and consequently may experience more significant quality of life deterioration with aggressive cancer treatment. It is unclear whether those frail patients would recover as reliably to their preoperative baseline or would suffer from persistently worse quality of life. As the authors acknowledge, this focus on healthier trial participants may limit the generalizability of these conclusions.
Additionally, careful consideration should be given to the response rates of the HRQOL study questionnaire. The authors conceded that more than a third of the patients lacked a baseline questionnaire: in the majority of cases, this was due to an administrative error. The authors make a reasonable case that since other baseline characteristics of the missing patients were similar to the overall cohort, their subsequent responses could and should be included. More worrisome, however, was that the response rates at later time points in the study were low enough to threaten the validity of the results. The response rate ranged from 58–62% for surgery alone patients and 69–75% for nCRT plus surgery patients. They specified that >10% of patients were “too ill” to complete the survey at each interval, and that >20% were “randomly missing” or missing for other reasons, though they do not specify how this determination was made or whether these individuals were evenly distributed in the nCRT and surgery alone groups. This large fraction of missing surveys due to severe illness suggests that the assessment of HRQOL may be an overestimate, due to a non-response bias. Also, the discrepancy in response rates between treatment groups calls into question whether HRQOL was truly equivalent: patients in the surgery alone group completed the survey at lower rates than those in the nCRT group. One possible implication may be that the surgery alone group might have had more patients who became seriously ill or died at earlier time points, but the difference in HRQOL was missed because the sickest patients were not uniformly captured in the surveys. These are important considerations when interpreting the results of the survey.
Finally, the generalizability of this data to patients receiving different induction chemotherapeutics or higher doses of radiation remains unknown. The CROSS regimen used carboplatin and paclitaxel and 41.4 Gy of external beam radiation administered with 3D conformal radiation technique. There is some variability in chemotherapeutic agents used (4), and alternate regimens may have different side-effect profiles that may impact HRQOL differently. Also, doses of radiation higher than 41.4 Gy are frequently given since recent evidence has shown that higher doses are associated with higher pathologic complete response rates (5,6). Additionally, the emergence of intensity-modulated radiation therapy (IMRT) that focuses radiation on the tumor and minimizes toxicity to adjacent structures may improve the tolerability of induction radiation therapy. The results of this study may not be applicable to all neoadjuvant protocols.
Despite these limitations, the authors should be congratulated for contributing very detailed data on HRQOL after induction therapy treatment to the field. Improving patient survival has always been a primary goal of esophageal cancer treatment, but the quality of life remains important, too. Recently, the importance of quality of life after surgical treatment has become a larger focus for patients, and multiple national societies have recognized the importance of patient-reported outcomes (7). Expectations of post-treatment symptomatology and functioning should be a part of the conversation as patients and providers choose a treatment pathway together. This study provides important data to inform that counseling.
Furthermore, the equivalence of HRQOL outcomes seen in this study between patients getting nCRT and those getting surgery alone is a very important finding. If the survival benefit of nCRT seen in the original CROSS study had been shown here to be accompanied by a significant long-term negative impact on quality of life, then patients would need to consider the value of a longer life versus that of a better-quality life. This study demonstrates that improved survival does not come with a lasting cost in any measure of quality of life. This further strengthens the argument for use of induction chemoradiation followed by surgery as standard of care.
Acknowledgments
Funding: This work was supported by the Barnes Jewish Hospital Foundation, National Institutes of Health (NIH) (2T32HL7776-21), and the Division of Cardiothoracic Surgery at Washington University in St. Louis.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shan Gao (Department of Thoracic Surgery, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.06.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Noordman BJ, Verdam MGE, Lagarde SM, et al. Effect of neoadjuvant chemoradiotherapy on health-related quality of life in esophageal or junctional cancer: results from the randomized CROSS trial. J Clin Oncol 2018;36:268-75. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Lin D, Leichman L. The current status of neoadjuvant therapy for esophageal cancer. Semin Thorac Cardiovasc Surg 2014;26:102-9. [Crossref] [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [Crossref] [PubMed]
- Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg 2009;87:392-8; discussion 398-9. [Crossref] [PubMed]
- Khullar OV, Fernandez FG. Patient-Reported Outcomes in Thoracic Surgery. Thorac Surg Clin 2017;27:279-90. [Crossref] [PubMed]
Cite this article as: Semenkovich TR, Meyers BF. Understanding the impact of neoadjuvant chemoradiation on health-related quality of life in esophageal cancer. Video-assist Thorac Surg 2018;3:26.


