VATS lymph node dissection and staging: the Southampton experience
Introduction
The late Professor Tsugo Naruke [1934–2006] is considered to be the father of modern nodal dissection (1). His nodal map was the basis for the contemporary TNM8 International Association for the Study of Lung Cancer (IASLC) nodal map [2009] (2-5). Professor Naruke and his colleagues proposed the strategy of ‘lobe-specific” nodal dissection in 1999 (6). Invited to London in October 1996 for a meeting sponsored by the IASLC, he presented videos describing the technique of video-assisted thoracoscopic systematic nodal dissection (VATS-SND) (7). His videos inspired surgeons around the world (including the authors) to adopt his technique of nodal dissection (8). Since then there has been great interest in SND, extending the approach to access all stations (9-13).
There is a consensus that proper staging of the mediastinum is important in managing patients with non-small cell lung cancer (NSCLC) (14-17). The nodal status is needed to make informed decisions on adjuvant therapy. No preoperative investigation or procedure such as computed tomography (CT), positron emission tomography (PET) endobronchial ultrasonography (EBUS), esophagal ultrasonography (EUS) or mediastinoscopy can be compared with surgical lymphadenectomy (SND) (18,19). The closest to SND would be the lymphadenectomies performed through the transcervical approach [video-assisted mediastinal lymphadenectomy (VAMLA) and transcervical extended mediastinal lymphadenectomy (TEMLA)] (20-23). Whilst the controversy continues about VATS as a tool for comprehensive mediastinal nodal dissection, technological innovations such as Robotic surgery seems to add a little more to the existing technique of nodal dissection (24,25). Again the myths surrounding feasibility and safety of dissecting station 4L and 2L nodes, by VATS or open operation continue (9,26). The main impediment to complete nodal dissection on the left chest is recurrent laryngeal nerve (RLN) palsy. The devastating loss of voice (dysphonia) and swallowing (dysphagia) can be life threatening, especially considering that some of these patients are high risk, with limited pulmonary reserves.
In this observational descriptive study, we hope to describe how VATS mediastinal nodal dissection can be comprehensive and consistently safe. We will discuss challenges to VATS-SND and ways to harvest nodes in stations 4L and 2L with special reference to avoiding RLN palsy.
Methods
This is an observational descriptive review of operative notes and video recordings of patients referred for NSCLC surgery between 2007–2017. Most of these patients had a confirmed or presumed diagnosis of NSCLC. These patients were operated on by VATS major pulmonary resection (VMPR) and routine SND even if nodal involvement was not suspected on 18FDG PET-CT (27-30). Patients with single zonal N1 disease were included from the beginning of the programme in 2005, and later in the series, single zonal N2 cases were added. The American College Of Surgery Oncology Group (ACOSOG) Z0030 trial has defined SND as removal of at least two lymph nodes from at least three mediastinal stations and should include stations 2R, 4R, 7, 8, and 9 for right-sided cancers and stations 4L, 5, 6, 7, 8 and 9 for left-sided cancers (31). At least 95% of patients should have 10 nodes harvested on average. We adhere to this strategy. Early in our series, 4L and 2L were not harvested routinely due to their proximity to the RLN. To improve our technique, thorough understanding of the anatomy of the RLN was obtained by cadaveric dissection of freshly embalmed humans at the All India Institute of Medical Sciences. We now consistently and safely harvest 3a, 3p and 2–4R on the right, and 5–6, 4L and 2L on the left (32,33). Our technique has evolved over the years from using the unipolar diathermy and ultrasonic energy devices into using a bipolar articulating diathermy device; EnsealTM (Johnson & Johnson, Ethicon, Cincinnati, USA). The impact on phrenic and RLN palsy was subjectively noticeable.
We do not transect the Azygos arch or the ligamentum arteriosum routinely. However; transection of the ligamentum arteriosum was required in selected patients to improve access to large 4L nodes. It is interesting to note that the European Society of Thoracic Surgeons (ESTS) advocated routine transection of the ligamentum arteriosum to mobilize the aorta and obtain nodes in station 4L (34).
Results and discussion
Between 2007–2017, we operated on 600 patients (240 left side, 40%). VATS-SND was completed as routine part of VMPR even if nodal involvement was not suspected on PET-CT scan.
Where do these nodes hide?
This description is based on the IASLC Mediastinal nodal map 2009 (4). The expected number of nodes is based on a cadaveric study by Ziyade et al. 2013 and Toker et al. 2011 (Table 1) (10,35).
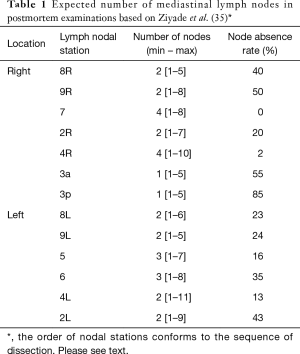
Full table
Of note, nodes in group 1 lie above the clavicle and as such are not approachable by VATS. Information about stations 12, 13 and 14 are obtained from the histopathology laboratory, as these lie deep within the removed part of the lung. Mediastinal nodal dissection usually pertains to N2 nodes, N1 hilar nodes (station 10) and interlobar N1 (station 11) nodes. All mediastinal nodes are covered by the investing medial pleura, and this has to be opened to gain access to the nodes.
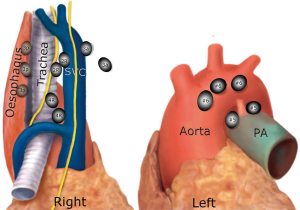
Right side (from bottom to top) (Figure 1)

Group 8 (para-oesophageal nodes): these are found lateral to the oesophagus on its infra-azygos course, down to the diaphragm. They are easily confused with station 9, station 7, and paratracheal station 10L. They can be difficult to access and harvest in the presence of a large hiatus hernia. Structures at danger are the trunk of the vagus nerve and the oesophagus. If the latter is not mobilized, the thoracic duct is not at risk. Expected median number of nodes [range] =2 [1–5], the possibility of absent nodes =40%.
Group 9: these are found within the folds of the inferior pulmonary ligament, from the inferior pulmonary vein down to the diaphragm. Nodes found high around the inferior pulmonary vein should be harvested separately from the lung specimen in case of lower lobectomy. It might be tempting to leave them attached to the vein when removing the lower lobe; however, the pathology lab might not realize the importance of these nodes and might report them as N1 (hilar nodes), whereas they are in fact N2 nodes. This significantly migrates the staging. Expected median number of nodes [range] =2 [1–8], the possibility of absent nodes =50%.
Station 7 (subcarinal nodes): this is the only median nodal group, exactly the same on the right as on the left side. Nodes are found between the right and left main bronchi at their origin and as low as the lower border of the bronchus intermedius. The oesophagus is posterior to this space and the pericardium is anterior. These nodes receive their arterial blood supply from bronchial arteries arising directly from the aorta, and bleeding here can be notorious. The space between #7 nodal pack and the pericardium is relatively avascular and low risk for starting the dissection. When close to the oesophagus, care must be taken not to dig holes in this structure. The same goes for the nearby membranous part of the bronchus. The right vagus nerve lies posterior to the hilum, on the lateral wall of the oesophagus, and sends small sensory twigs to the bronchi and the nodal pack. All these vagal bronchial branches could be cut in the process of exposing the subcarinal space. However; there is no impunity in cutting the vagal trunk, as this might result in delayed gastric emptying and bloating. The subcarinal space should be well displayed by the end of this dissection, and the left main bronchus should be readily identifiable. The IASLC map has extended the position of subcarinal #7 nodes to the lower limit of the bronchus intermedius (upper border of the lower lobe bronchus), which means that parabronchial nodes on the bronchus intermedius, previously labeled as 10R are now considered as station 7. Para-oesophageal 8R and contralateral paratracheal 10L nodes on the left main bronchus are also close by. As long as the oesophagus is not mobilized, the Thoracic duct is tucked out of harm’s way between the oesophagus and the vertebral bodies. Expected median number of nodes [range] =4 [1–18], the possibility of absent nodes = approaching 0% (almost always there).
Stations 2&4R: this fibro-fatty-nodal pack lies within the ‘Superior Triangle’ made by the phrenic nerve, the vagus nerve and the Azygos arch (blue blood; same as left side). There is no physical boundary between 2R and 4R nodes, and the pack is usually harvested en bloc. There is absolutely no need to transect the Azygos arch to harvest this pack of nodes (11). The IASLC map has shifted the median line to the left border of the trachea. Technically station 2R nodes are found on the right lateral wall of trachea, anterior to it (pretracheal) and up to the left border of the trachea. On the right, the IASLC map describes the lower border of 2R as “intersection of the caudal margin of innominate vein with the trachea”. This is not readily identifiable by the surgeon as shown in Figure 1, and a better landmark would be the origin of the brachiocephalic trunk from the aortic arch. Also note that these landmarks are seen only after the nodal pack has been harvested, and not before. This controversy of territorial landmarks was described before by Ichimura et al. (37). Some lymph nodes will overlap the borders between lymph node levels no matter how these are defined and will need to be labeled according to where they predominantly reside (38). Separate identification of 2R from 4R nodes might be required for “lobe-specific” strategy. This can be achieved by dividing the nodal pack into two halves, and the top one can be labeled as 2R nodes. We do not separate 2R and 4R routinely, as their involvement carries the same significance of N2 disease, and the need of adjuvant chemotherapy. Heterogeneity of N2 disease is not taken into great consideration in the literature when it comes to the need for adjuvant therapy (39). It must be mentioned however that involvement of the higher 2R nodes carries worse prognosis than 4R. For the right lower paratracheal lymph nodes (station 4R), the lower border is the lower margin of the azygos vein. Station 4R are found in many locations, namely, lower right paratracheal, lower pretracheal (up to left border of trachea) and retrocaval precarinal. Nodes below the azygos arch are hilar 10R nodes.
When all 2&4R nodes have been harvested, the base of the superior triangle becomes evident. It is made of the arch of the aorta and its branch the brachiocephalic trunk. Exposing the aortic arch and brachiocephalic trunk is a surrogate index of completeness of nodal dissection of this group. The nodal pack is supplied by small arteries directly arising from the arch of aorta and brachiocephalic artery. Venous return is direct from the pack to the back of the SVC via a constant short vein. At the apex of this triangle, one large lymphatic channel can be seen cursing up the lateral border of the SVC and innominate vein, to join the jugulo-subclavian venous confluence posteriorly. This is the counterpart of the thoracic duct and is dubbed the right thoracic duct. It is more translucent and lobulated than a nerve, and not constantly found. In theory, injury of this large lymphatic channel could lead to postoperative chyle leak. The main danger of harvesting 2&4R at the apex of the superior triangle is RLN injury. Thermal injury is almost always the reason, as the nerve is tightly tucked around the subclavian artery, and so disconnection would be an unlikely cause. A disconnecting insult would certainly cause some significant bleeding at this location. Expected median number of 2R nodes [range] =2 [1–7], possibility of absent nodes =20%. Expected median number of 4R nodes [range] =4 [1–10], the possibility of absent nodes =2%.
Group 3a and 3p: These two groups are outside the superior triangle. 3a nodes are prevascular, anterior to the SVC between the phrenic nerve laterally and the sternum medially. They are sometimes clearly seen under the pleural investment. This area between the SVC and the sternum can contain part of the thymus gland. Not routinely harvested, but if seen to exist, 3a nodes are worthwhile including in the dissection. Expected median number of 3a nodes [range] =1 [1–5], the possibility of absent nodes =55%.
3p nodes are retro-tracheal, between trachea and oesophagus, above the Azygos arch. These nodes lie within a mesh made by sensory branches of the vagus and the RLNs at the point of looping around the subclavian artery. Again this group is not harvested routinely, but PET activity should mandate their clearance. Expected median number of 3p nodes [range] =1 [1–5], the possibility of absent nodes =85%.
Left side (from bottom to top) (Figure 2)

Group 8L (para-oesophageal nodes). Again, these are found lateral to the oesophagus between the descending aorta and pericardium, at a level below the upper border of the left lower lobe bronchus and down to the diaphragm. A better landmark is the pulmonary vein to diaphragm. One has to be aware of structures that are usually not there but can be there, such as a hiatus hernia. Expected median number of 8L nodes [range] =2 [1–5], the possibility of absent nodes =24%.
Group 9L: similar to the right side, found within the folds of the inferior ligament. Again all 9L nodes must be harvested separately, lest they are misreported as N1 nodes. Expected median number of 9L nodes [range] =2 [1–6], the possibility of absent nodes =23%.
Group 7 (subcarinal nodes): this is exactly the same group described on the right, however; exposure from the left side is trickier and takes a longer time. These nodes are found at the third tier of depth, deeper than the oesophagus, which is deeper than the descending aorta. It is to be noted that the descending aorta, oesophagus and the right main bronchus are stacked on top of each other in one axis. Clear preparation of the back of the hilum is crucial for exposure of #7 nodes. All the vagal bronchial branches at the back of the hilum, together with the bronchial arteries directly from the descending aorta must be disconnected first. The left main bronchus is followed proximally until the right main bronchus is clearly exposed. Earlier in our experience, we used to pass a sturdy tape around the left main bronchus to retract it anteriorly, which seems to bring the deep subcarinal space forward and make the dissection easier. Expected median number of nodes [range] =4 [1–18], the possibility of absent nodes = approaching 0% (almost always there).
Group 5&6: these two groups are found on the left only. They share some characteristics with the right 2&4R insofar as they are found within a superior triangle made by the vagus and phrenic nerves, and based on the left main pulmonary artery (blue blood). Likewise, they are usually harvested en bloc, and their clearance is important to expose the RLN. It is therefore recommended to harvest them before 4L nodes. Group 6 are prevascular on the arch of the aorta, between the highest and lowest borders of the Arch. The superior intercostal vein crosses the arch of aorta (and the apex of the triangle) from left to right to join the innominate vein. The nodes lie over the vagus and RLN, which are at risk of thermal injury. On average there are 3 (range, 1–7) and they are absent in 35% of cases. Group 5 are found at the concavity of the aortic arch, between it and the pulmonary artery (sub-aortic space). These nodes lie lateral to the ligamentum arteriosum, which is not seen unless #5 nodes are cleared. They get their blood supply directly from the arch of the aorta and receive sensory nerves directly from the vagus and RLN. They can easily be confused with 4L nodes, which are found medial to (and hidden by) the ligamentum. The average number of nodes is 3 nodes (range, 1–8) and they are missing in 16%. The main danger of dissecting group 5&6 is injury to the phrenic nerve, and less so to the RLN. We, therefore recommend slinging the phrenic nerve routinely to be able to track its course. Anterior to the phrenic nerve, the fibro-fatty-nodal pack merges seamlessly with the thymic fat, such that overzealous dissection will obtain thymic tissue.
Group 4L: includes nodes to the left of the left lateral border of the trachea, medial to (and hidden by) the ligamentum arteriosum. The upper border is the upper margin of the aortic arch and lower border is the upper rim of the left main pulmonary artery (4). There is a constant inferior cardiac branch of the vagus nerve that traverses the superior triangle, and this can be cut with impunity. Interestingly the same nerve traverses the right superior triangle, and is similarly treated. At the reflection point of the RLN, both the vagus and its recurrent branch give multitude of short sensory twigs to the surrounding structures, such as the pulmonary artery, trachea, oesophagus and nodal tissue. Interestingly this organized chaos of sprouting sensory nerves exist bilaterally at the same reflection point of the RLN. Lymph nodes are trapped within these twigs akin to fish trapped in a fisherman’s net. These nerves act like guy ropes to fix the point of reflection. For this reason, the RLN should not be retracted or slung to avoid traction injury. The surgeon must locate and track the motor branch of the RLN, which curses to the right of the reflection point (not left!), heading up to the tracheo-oesophageal groove. All other sensory twigs could be safely cut in the process of exposing 4L nodes. The nodes in juxtaposition to the motor RLN are grabbed with a node forceps and teased out slowly without using any energy-spraying device at all. One should resist the temptation of using monopolar diathermy to control bleeding close to the RLN, pressure is all that is needed. For large nodes and 18FDG-avid nodes on the PET, it is preferred to staple the ligamentum arteriosum to improve access. Care must be taken to assess the risk of bleeding from a heavily calcified ligamentum before proceeding with stapling it. In general it is safe to do that, and this is the recommendation of the European Society of Thoracic Surgery (34). Expected median number of nodes [range] =2 [1–11], the possibility of absent nodes =13%.
Group 2L: these are found in a tight diamond shaped space between the subclavian and carotid arteries. The lower border of this space is the superior border of the aortic arch, and its upper border is the first rib. The space is a continuation of the paratracheal space that contains #5 and 4L nodes. Where covered by the aortic arch, the paratracheal space contains 4L nodes, and above the Arch it contains 2L nodes. The descending vagal trunk runs in this space in a superficial plane just under the pleura. It descends lateral to the carotid artery when entering the thoracic inlet, and then crosses the space laterally over the origin of the subclavian artery, and under the superior intercostal vein. The arrangement of the origin of the subclavian and left common carotid arteries from the aortic arch might render harvesting this group a bit challenging, however these large arteries are quite mobile and can safely be retracted to expose the space for nodal harvesting. The main stem trachea is readily recognized between the two arteries. Nodes in this area are easily mixed with 3a nodes, which lie in a prevascular plane, between the carotid artery and the sternum, far from a paratracheal position. These can be harvested before embarking on 2L nodes to improve exposure. There are three potential dangers in this small apical diamond-shaped space; the descending vagal trunk, the ascending RLN and the thoracic duct. Inadvertent disconnection or thermal injury of the descending vagal trunk will cause RLN palsy, as the RLN is contained within its sheath in this location (two bananas in one skin). The ascending RLN on the other hand, is found at a deeper level. The subclavian artery can be taken as a surrogate land mark to the trachea-oesophageal groove in which the nerve lies. Most importantly the surgeon should be aware of the thoracic duct, which takes a short course behind the middle point of the subclavian artery, heading medially across the space to the back of the subclavian/innominate vein to join the jugulo-subclavian venous confluence. Expected median number of nodes (range) =2 [1–9], the possibility of absent nodes =43%.
What is a nodal zone?
A nodal zone is an anatomical area that includes one or several neighbouring N2 nodal stations (41). Nodal zones help in locating nodal involvement without having to define the exact anatomical location of the nodes. The supraclavicular (station 1) and the subcarinal zones (station 7) include one nodal station each, whereas other nodal zones include two, three or more nodal stations. For example the right paratracheal area (single zone) contains stations 2R, 4R, 3a and 3p (Figure 3). It is important to realize that, in theory, a single zone may have one or multiple nodes involved in one or more nodal stations, and that the nodes may be small or large. The concept of nodal zones is of special value for those patients who will receive chemotherapy or radiotherapy as their definitive modality of treatment. The precise anatomical location of the nodes involved is not so important.
Strategies for mediastinal staging
There are different protocols for staging the mediastinum:
- Selective nodal sampling: the surgeon decides which node(s) looked diseased and randomly removes that node (chance node).
- Sentinel nodal sampling: at operation, the primary tumour is injected with 99Technitium tracer, and a Geiger counter is used to identify the sentinel hilar nodes, which are then removed. If frozen section has confirmed the absence of metastases, the rest of nodal dissection is omitted. Assessment of sentinel nodes proves useful in staging patients with melanoma and breast cancer (decision node) (42). It is becoming important in segmentectomy and subsegmentectomy for Ground Glass Opacities which prove to be NSCLC.
- Systematic nodal sampling: one or two nodes sampled from each zonal station, total of at least 6 nodes (selective) (31,43). Station 7 could be omitted.
- SND: at least two nodes from each field or station, and at least three fields are dissected (total of at least six nodes). One must always include subcarinal nodes (universally accepted). The surgeon’s whole series has to be consistent insofar as 95% of patients should have more than ten nodes harvested (31).
- Lobe-specific nodal sampling: suggested by Asamura and Naruke, this strategy is oriented towards the different lymphatic drainage of different lobes, and advocating that the most distant nodal groups from the lobe in question, need not be harvested. Subcarinal lymphadenectomy is not always necessary for tumours of the right upper lobe and left upper trisegmentectomy (selective) (6,44). At least six nodes are taken in total. For right upper or middle lobectomy 2R, 4R and #7 are harvested, excluding #8R & 9R. For right lower lobectomy 4R, #7, 8R, 9R are harvested, excluding #2R. For left upper lobectomy stations 5, 6, 7 are harvested, excluding #2L, 4L, 8L, 9L. For left lower lobectomy #7, 8L, 9L are harvested, excluding #2L, 4L, 5, 6. Note the exclusion of dissection of #2L and #4L in all cases, perhaps a reflection of the technical abilities at that time [1999].
- Extended nodal dissection: by definition means bilateral mediastinal lymphadenectomy through a median sternotomy (no consensus on extent, this strategy has questionable morbidity) (45,46).
The most popular three protocols are SND, systematic nodal sampling (SNS) and lobe-specific sampling (LSS). The randomized controlled trial Z0030 has shown no statistical difference in overall survival between SND and SNS (43). Patients with fewer than six lymph nodes harvested have a significantly worse survival than matched patients with greater than 6 lymph nodes despite ostensibly having the same pathologic stage (47,48). Hence the recommendation in the 7th edition of the AJCC/UICC staging guidelines for examination of at least six lymph nodes and three nodal stations (3).
How to avoid damage to the RLNs (Figure 4)

Complete nodal dissection has been historically hampered by the technical inability to harvest stations 4L (left lower paratracheal), 2L (left upper paratracheal), station 5 (subaortic) station 6 (preaortic), station 3p (right retrotracheal) and station 2&4R (high and low right paratracheal, pretracheal and precarinal). The main impediment to achieving this goal is the devastating complication of dysphonia and dysphagia due to RLN damage. The recent development of routine systematic mediastinal nodal dissection (SND) during surgery for NSCLC has seen a surge in the incidence of RLN palsy (50).
Absolute mastery of the anatomy of the RLN in the chest is mandatory for its preservation. In the chest, the course of the right RLN is short and damage is exceptionally rare. The left RLN runs a more complex course and requires in-depth understanding. Despite the complex interaction of the left RLN with station 4L nodes, it is consistently possible to harvest this group of nodes with safe results. The ligamentum arteriosum can safely be cut in selected cases, to improve exposure of 4L nodes. In this series there were no RLN palsies on the right side and 3 on the left side (1.3%). None of the 3 RLN palsies were recorded in the operation notes, suggesting the surgeon was unaware of injury at the time of operation. Two of the RLN palsies were due to thermal injury (monopolar diathermy) and one to inadvertent transection of the descending vagal trunk. All RLN palsies were diagnosed in the immediate postoperative period. One patient with thermal injury had spontaneous improvement within 6 months and did not require intervention. The other two patients underwent medialization laryngoplasty, resulting in near normal voice. There were no RLN palsies attributable to energy spray after changing practice from monopolar to bipolar diathermy. We firmly believe that the best way to avoid injury to the RLN is to expose it and preserve its motor branch. We also found the use of a bipolar device to be safe up to 1 millimeter away from the motor RLN. Future studies, similar to those undertaken during thyroidectomy, are awaited, whereby patients are randomized to SND with Intraoperative nerve monitoring (IONM) to verify RLN function integrity, versus SND relying on visualization alone and the surgeon’s knowledge of the anatomy of the nerve (51).
Across midline harvesting of nodes (Figure 5)

VATS mediastinal nodal dissection is designed to harvest nodes unilaterally. However; it is possible to gain access to some of the lymph nodes across the median line, without the need for median sternotomy. Figure 1 has already shown access to 10L nodes during dissection of the subcarinal nodes from the right side. The same is true for accessing station 10R from the left chest. These nodes are not far away from the midline, and careful reading of the CT scan will allow determining an access strategy from the contralateral side. Figure 5 demonstrates how one can gain access to 4L nodes from a right VATS approach, and per contra, how to access 4R from a left approach. The first group of nodes is approached by developing a plane between the main trachea and the oesophagus, above the Azygos arch. Dissection of stations 2-4R makes the exposure easier, but this is not mandatory. It is also possible to biopsy some station 4R (pretracheal) nodes from the left side as part of 4L dissection. These nodes will be anterior to the trachea, which is well exposed during 4L dissection. Standard exposure of the ligamentum arteriosum is achieved by removing stations 5&6, followed by appreciating the course of the motor branch of the RLN. Dissection within the pre-tracheal plane is possible and can be improved by transecting the ligamentum, but again that is not mandatory.
Can VATS mediastinal nodal dissection be as comprehensive as thoracotomy?
General skepticism about VATS or specific lack of experience with VATS nodal dissection might fuel the argument that open thoracotomy could be superior to VATS in harvesting nodes. Indeed Zhang et al. pointed out that open thoracotomy is a better way of performing mediastinal nodal dissection (53). Their meta-analysis showed that fewer total lymph nodes were dissected by VATS compared to open thoracotomy. The Danish experience [2013] seems to agree with this sentiment (54). On the other hand Watanabe et al. [2005] have demonstrated SND by VATS was not inferior to that through an open thoracotomy (55). In our opinion surgical access to the nodal stations and the number of nodes harvested is a direct function of the surgeon’s experience and how much time is dedicated to the procedure. It is so clear in our minds that VATS should be the gold standard, and that it is possible to perform complete adenectomy should the surgeon wish to do so. In experienced hands, complete adenectomy on the right takes 30 minutes and on the left 45–60 minutes, which is consistent with the open thoracotomy experience reported by Weyant and Flores [2008] (11). We are struggling to fathom the ethicality of setting up a randomised controlled trial (RCT) between VATS and thoracotomy for SND, however when this was done by Palade et al [2013], there was good evidence to suggest that VATS is as effective as open thoracotomy (56). Furthermore, the video-assisted approach allows a better visualization of different lymph node zones.
VATS lobectomy first or nodal dissection first?
There are merits for starting either one first. In the case of a large tumour, it is best to start with the lung resection, to generate a working space after retrieval of the lung specimen. However, if space is not at a premium, then there is merit in starting with nodal dissection. Hilar circumcision and clearance of nodes make the lobar resection easier. In some post-induction cases it might lead the surgeon to abort a complex resection if the nodes confirm metastasis. On the left side, we use the intact lung to retract the pulmonary artery down. This maneuver opens the sub-aortic space and facilitates dissection of 4L nodes. It is our routine on the left side to attempt the nodes first.
Routine staging in CT/PET N0 patients, is it necessary?
Osarogiagbon et al. investigated the impact of omitting nodal assessment in patients with stage I NSCLC (CT and PET negative). They analyzed resections for first-time primary, pN0 NSCLC in the United States Surveillance, Epidemiology, and End Results (SEER) database from 1998 to 2009 (57). Patients who had no clinical evidence of nodal disease but were pNX (not assessed) had a 5-year survival similar to those with pN1 tumours, suggesting significant understaging at the time of operation. By not assessing the nodal state of our patients, there is a possibility that we might be condemning them to a prognosis similar to pN1 disease at best (15,57).
Preoperative versus operative staging of the mediastinum
The significance of preoperative as opposed to operative staging in resectable early lung cancer is tied to what the clinician wants to do with the information. There is evidence in the literature that postoperative adjuvant chemotherapy for NSCLC could improve the 5-year survival by 5% in the European series (14 countries), and by 11% in the Japanese studies (58-60). However; some argue that the evidence of mediastinal nodal involvement should be sought before the operation, and in case of N2 disease, the patient should be offered preoperative (neoadjuvant) chemotherapy. The follow-up to the S9900 trial, published in 2007, concluded that the best treatment for N2 resectable lung cancer would be induction chemotherapy followed by surgery (61). For such a strategy every effort is sought to prove or disprove N2 disease before the operation, as discovery of N2 nodes changes the clinical pathway altogether. Such urge for proper staging has led to a boost and expansion in the scope of EBUS to include a staging EBUS. Naur et al. concluded that the overall probability of upstaging by EBUS in patients judged as N0/N1 at PET-CT was 6.0% (62). However; these investigations seem to prolong the clinical pathway, especially if surgery is the final common pathway.
Two recent publications have polarized the main debate around mediastinal nodal dissection. The first was the revised ESTS guidelines [2014], which suggested that when there are no enlarged lymph nodes on CT and when there is no uptake in lymph nodes on PET, direct surgical resection with SND is indicated for tumours ≤3 cm located in the outer third of the lung (63). However; in central tumours or N1/N2 nodes, preoperative mediastinal staging is indicated, regardless of operability. The second publication was by Lim et al. [2015] in which they explained clearly why invasive mediastinal staging is irrelevant for PET-CT positive N1 or N2 lung cancer if the primary tumour and ipsilateral lymph nodes are resectable (64). Previously Lim et al. reported a systematic review of all the published meta-analysis of randomised trials in preoperative versus postoperative chemotherapy in patients with resectable lung cancer (65). There was no difference in the 5-year overall and disease-free survival between the timing of administration of chemotherapy pre or postoperatively. This justified a trend of operating on single zonal N2 disease followed by chemotherapy (66-68). We do believe that CT-negative PET-negative patients should go directly to operation with SND. We also agree with the reasoning that invasive mediastinal staging is irrelevant for PET-positive N2 lung cancer if the primary tumour and ipsilateral lymph nodes are resectable. Given that survival is similar for bimodality management (chemo/radiotherapy) and better for surgical multimodality management for patients with resectable N2 disease (surgery followed by chemotherapy), guidelines from the UK and the USA already advocate the role of surgery for selected patients with N2 disease (17,66,68). However; data to support these choices are awaited.
We rarely restage at our institution, however; indications for post-treatment nodal biopsy are expanding hand in hand with the expansion of targeted therapy. Tyrosine kinase inhibitors (TKIs) that can treat lung cancers with EGFR, ALK or PD-L1 mutations have recently been successful. Most of the requests in our institution for post-treatment nodal sampling would be to obtain tissue for mutation studies. Repeat mediastinoscopy is used only in very selected experienced centres and is not widely adoptable due to severe fibrosis (69,70). If needed, we prefer VATS for restaging, as dealing with operative adhesions is more controllable and likely to succeed (Figure 6). Sometimes postoperative 18FDG uptake in a nodal group is not necessarily indicative of local recurrence. Figure 7 shows the CT and PET of such a patient. This was the only 18FDG avid lesion in the body, 2 years following VATS right upper lobectomy and SND. We were asked to revisit the nodes at 2 & 4R to justify local radiotherapy. On VATS reoperation the superior triangle was reopened to find the alleged recurrence to be a collection of sterile pus. This is a good example that ‘not all that glitters is gold’.
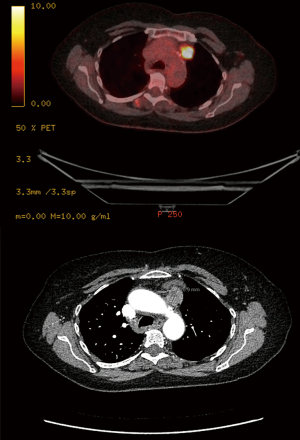
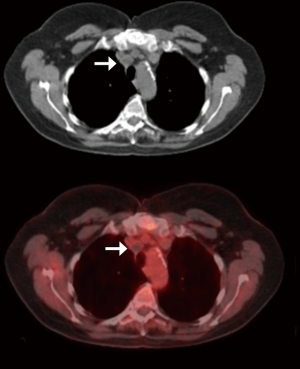
Unfortunately, VATS-SND is not accepted as a safe preoperative investigation, whereas other even more invasive techniques such as VAMLA and TEMLA were recognized as so. Whether the adhesions from the procedure are thought to preclude the primary surgical resection remains speculative. Again the emphasis should be on what the clinician wants to do with the information. At Southampton, our practice has evolved to operate on single zonal N1 and single zonal N2 disease. There is no pressing necessity to have tissue confirmation of nodal disease before the operation as long as the lesion remains operable. For us, SND will stay an integral part of the VATS major pulmonary resection, and not a preoperative investigation.
Complications and challenges to SND (Figure 8)

From our 600 cases, there was not a single case of postoperative chylothorax. There were no complications attributable to transection of the ligamentum arteriosum (4 cases). There were no complications to disconnecting the vagal sensory branches, such as bradycardia, malignant arrhythmia, dysphagia or dysphonia. There were four cases of inadvertent bleeding directly related to SND. Blood loss in three of these cases exceeded 500 mills. One right sided bleeding involved the SVC and required immediate conversion to thoracotomy. Two small bronchial artery bleeders caused a significant enough haemothorax to warrant re-exploration by VATS on the first postoperative day. One pinhole bleeding at the medial wall of the Azygos arch required stapling and disconnection of the Azygos vein.
Three phrenic nerve palsies are reported, one on the right and two left-sided, all due to monopolar diathermy. There was no RLN palsy on the right side and 3 on the left side (1.3%) as previously discussed. There was no RLN palsy attributable to energy spray after changing practice from monopolar to bipolar diathermy.
There were three cases of port-access site metastases. Retrieval bags were not used to extract the nodes in these patients. Nevertheless; none of the patients had nodal involvement by tumour, and mechanical contamination of the port-sites cannot explain the seedlings. A humoral factor has to be presumed.
There are few challenges to SND that we encountered during our experience. Congenital absence (agenesis) of the pericardium was one of those (72). The exposed heart makes dissection of the para-oesophageal #8L nodes very difficult, if not impossible. The reason for that is the inability to retract on the heart for the fear of injuring the epicardial coronary arteries. In addition, introduction of instruments through ports has to be extremely careful, as accidental scratching on the heart surface carries the risk of coronary artery injury and subsequent intraoperative myocardial infarction.
Sometimes large malignant nodes could be adherent to structures such as the trachea or oesophagus in a way that makes their removal unwise. Serious complications such as perforation of the oesophagus or trachea are to be avoided at all costs. The risks to patient should be assessed and reassessed continuously, and sometimes objectives have to be abandoned for safety reasons.
Total adenectomy rather than a sampling strategy is indicated for patients with NSCLC and a past or concurrent history of lymphopoietic diseases such as lymphoma or chronic lymphoid leukaemia. Reactivation of their disease is always a possibility and nodal adenectomy gets the staging spot on. Reliance on the PET scan for the staging would significantly migrate the staging in these dual pathologies.
Conclusions
In conclusion, cumulative experience in a large throughput centre improves the safety record of VATS comprehensive mediastinal nodal dissection. VATS allows the surgeon to access all nodal stations consistently, safely and with minimal complications. A better understanding of the RLN anatomy and the use of bipolar energy devices have contributed to the significant drop in phrenic and RLN palsies in our experience.
Acknowledgments
We are indebted to Dr. George Karimundackal, consultant oncological surgeon at Tata Hospital, Mumbai, India for his generous and valuable contributions. We are also very grateful to Professor Harushi Osugi, Department of Surgery, Institute of Gastroenterology, Tokyo Women’s Medical University, Kawada-Cho, Shinjuku-ku, Japan for providing a valuable video clip about three field nodal dissection during VATS oesophagectomy. Cadaveric dissections were performed at the All India Institute of Medical Sciences, which gave the team the golden opportunity to understand the detailed anatomy of the RLNs.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Todd Demmy) for the series “VATS for Locally Advanced Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.04.05). The series “VATS for Locally Advanced Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients were consented appropriately and permission to video-record their operation was obtained. The VATS lobectomy Programme including Systematic Nodal Dissection was approved by our local Clinical effectiveness committee in 2005, which included ethical approval. Cadaveric dissection was undertaken in accordance with the ethical standards of the All India Institute of Medical Sciences.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Asamura H. In memoriam. J Thorac Oncol 2007;2:261-2. [Crossref]
- Naruke T, Suemasu K, Ishikawa S. Lymph node mapping and curability at various levels of metastasis in resected lung cancer. J Thorac Cardiovasc Surg 1978;76:832-9. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project. A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Eberhardt WE, Mitchell A, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2015;10:1515-22.
- Asamura H, Nakayama H, Kondo H, et al. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg 1999;117:1102-11. [Crossref] [PubMed]
- Goldstraw P. Report on the International Workshop on Intrathoracic Staging. London, October 1996. Lung Cancer 1997;18:107-11. [Crossref]
- Naruke T, Tsuchiya R, Kondo H, et al. Lymph node sampling in lung cancer: how should it be done? Eur J Cardiothorac Surg 1999;16:S17-24. [Crossref] [PubMed]
- Toker A, Kaya S, Erus S, et al. Dissection of Superior Mediastinum in Patients with left sided Hilar Lung Cancer. 2010. Available online: https://www.youtube.com/watch?v=UJJofZaZnNA, last accessed 19.04.2018
- Toker A, Tanju S, Ziyade S, et al. Alternative paratracheal lymph node dissection in left sided hilar lung cancer patients: Comparing the number of lymph nodes dissected to the number of lymph nodes dissected in right sided mediastinal dissections. Eur J Cardiothorac Surg 2011;39:974-80. [Crossref] [PubMed]
- Weyant M, Flores R. VATS Mediastinal Nodal Dissection. 2008. Available online: https://www.ctsnet.org/article/vats-mediastinal-nodal-dissection, last accessed 30.09.2017.
- Reichert M, Steiner D, Kerber S, et al. A standardized technique of systematic mediastinal lymph node dissection by video-assisted thoracoscopic surgery (VATS) leads to a high rate of nodal upstaging in early-stage non-small cell lung cancer. Surg Endosc 2016;30:1119-25. [Crossref] [PubMed]
- American College of Surgeons Clinical Research Program, Alliance for Clinical Trials in Oncology, Heidi D, et al. Operative Standards for Cancer Surgery Volume 1. China: Wolters Kluwer, 2015:123-34.
- Nohl HC. An investigation into the lymphatic and vascular spread of carcinoma of the bronchus. Thorax 1956;11:172-85. [Crossref] [PubMed]
- Osarogiagbon RU, Decker PA, Ballman K, et al. Survival Implications of Variation in the Thoroughness of Pathologic Lymph Node Examination in American College of Surgeons Oncology Group Z0030 (Alliance). Ann Thorac Surg 2016;102:363-9. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-e250S.
- Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e314S-e340S.
- Al-Sarraf N, Gately K, Lucey J, et al. Lymph node staging by means of positron emission tomography is less accurate in non-small cell lung cancer patients with enlarged lymph nodes: Analysis of 1145 lymph nodes. Lung Cancer 2008;60:62-8. [Crossref] [PubMed]
- Van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of PET in the preoperative assessment of patients with suspected non-small cell lung cancer: The PLUS multicenter randomised trial. Lancet 2002;359:1388-93. [Crossref] [PubMed]
- Zielinski M, Szlubowski A, Kołodziej M, et al. Comparison of endobronchial ultrasound and/or endoesophageal ultrasound with transcervical extended mediastinal lymphadenectomy for staging and restaging of non-small-cell lung cancer. J Thorac Oncol 2013;8:630-6. [Crossref] [PubMed]
- Yendamuri S and Demmy T. Transcervical Extended Mediastinal Lymphadenectomy. 2011. Available online: https://www.ctsnet.org/article/transcervical-extended-mediastinal-lymphadenectomy, last accessed 01.10.2017
- Hürtgen M, Friedel G, Toomes H, et al. Radical video-assisted mediastinoscopic lymphadenectomy (VAMLA) - technique and first results. Eur J Cardiothorac Surg 2002;21:348-51. [Crossref] [PubMed]
- Kuzdzał J, Zieliński M, Papla B, et al. Transcervical extended mediastinal lymphadenectomy--the new operative technique and early results in lung cancer staging. Eur J Cardiothorac Surg 2005;27:384-90. [Crossref] [PubMed]
- Sagawa M, Sato M, Sakurada A, et al. A prospective trial of systematic nodal dissection for lung cancer by video-assisted thoracic surgery: can it be perfect? Eur J Cardiothorac Surg 2005;27:384-90; discussion 390. [PubMed]
- Toker A, Özyurtkan MO, Demirhan Ö, et al. Lymph Node Dissection in Surgery for Lung Cancer: Comparison of Open vs. Video-Assisted vs. Robotic-Assisted Approaches. Ann Thorac Cardiovasc Surg 2016;22:284-90. [Crossref] [PubMed]
- Nagashima T. Thoracoscopic left mediastinal lymph node dissection. Ann Transl Med 2016;4:10. [PubMed]
- Amer K, Khan AZ, Vohra HA. Video-assisted thoracic surgery of major pulmonary resections for lung cancer: the Southampton experience. Eur J Cardiothorac Surg 2011;39:173-9. [Crossref] [PubMed]
- Amer K, Khan AZ, Singh N, et al. Video-assisted thoracic surgery systematic mediastinal nodal dissection and stage migration: impact on clinical pathway. Eur J Cardiothorac Surg 2011;40:1474-81. [PubMed]
- Amer K. Thoracoscopic mediastinal lymph node dissection for lung cancer. Semin Thorac Cardiovasc Surg 2012;24:74-8. [Crossref] [PubMed]
- Khalid Amer (February 15th 2012). Video-Assisted Thoracic Surgery (VATS) Systematic Mediastinal Nodal Dissection, Topics in Thoracic Surgery Paulo Cardoso, IntechOpen, DOI:
10.5772/27676 . Available online: https://www.intechopen.com/books/topics-in-thoracic-surgery/video-assisted-thoracic-surgery-vats-systematic-mediastinal-nodal-dissection - Darling GE, Allen MS, Decker PA, et al. Number of Lymph Nodes Harvested from a Mediastinal Lymphadenectomy: Results of the Randomized, Prospective ACOSOG Z0030 Trial. Chest 2011;139:1124-9. [Crossref] [PubMed]
- Amer K. The Recurrent Laryngeal Nerves and the Thoracic Surgeon. CTSNet, Inc.2017. Available online: https://doi.org/
10.25373/ctsnet.5345176 Retrieved: 20:16, Sep 19, 2017 (GMT). - Amer K. The Recurrent Laryngeal Nerves and the Thoracic Surgeon. 2017. Available online: https://www.youtube.com/watch?v=gOcSaWRmZFA, last accessed 01 October 2017.
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non- small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Ziyade S, Pinarbasili NB, Ziyade N, et al. Determination of standard number, size and weight of mediastinal lymph nodes in postmortem examinations: reflection on lung cancer surgery. J Cardiothorac Surg 2013;8:94. [Crossref] [PubMed]
- Amer K, Khan AZ, Parshad R, et al. VATS nodal dissection of right mediastinal nodes, using the bipolar device EnsealTM. Asvide 2018;5:568. Available online: http://www.asvide.com/article/view/25396
- Ichimura H, Kikuchi S, Ishikawa H. Caudal border of level 2R in the new international lymph node map for lung cancer. J Thorac Oncol 2010;5:579-author reply 579-80. [Crossref] [PubMed]
- Rusch VW, Asamura H, Goldstraw P. Response to Letter to the Editor. J Thorac Oncol 2010;5:579-80. [Crossref] [PubMed]
- Rami-Porta R. Quantification of regional lymph node involvement in lung cancer. Thorax 2011;66:271-2. [Crossref] [PubMed]
- Amer K, Khan AZ, Parshad R, et al. VATS nodal dissection of left mediastinal nodes, using the bipolar device EnsealTM. Asvide 2018;5:569. Available online: http://www.asvide.com/article/view/25397
- Pisters KM, Darling G. The IASLC Lung Cancer Staging Project: “The Nodal Zone J Thorac Oncol 2007;2:583-4. [Crossref] [PubMed]
- Schmidt FE, Woltering EA, Webb WR, et al. Sentinel nodal assessment in patients with carcinoma of the lung. Ann Thorac Surg 2002;74:870-4; discussion 874-5. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Adachi H, Sakamaki K, Nishii T, et al. Lobe-Specific Lymph Node Dissection as a Standard Procedure in Surgery for Non-Small Cell Lung Cancer: A Propensity Score Matching Study. J Thorac Oncol 2017;12:85-93. [Crossref] [PubMed]
- Hata E, Miyamoto H, Tanaka M, et al. (The necessity of extended systemic dissection of the regional lymph node in radical operation for lung cancer). Kyobu Geka 1994;47:40-4. [PubMed]
- Sakao Y, Miyamoto H, Yamazaki A, et al. The spread of metastatic lymph nodes to the mediastinum from left upper lobe cancer: results of superior mediastinal nodal dissection through a median sternotomy. Eur J Cardiothorac Surg 2006;30:543-7. [Crossref] [PubMed]
- Osarogiagbon RU, Yu OO. Number of lymph nodes associated with maximal reduction of long-term mortality risk in pathologic node-negative non-small cell lung cancer. Ann Thorac Surg 2014;97:385-93. [Crossref] [PubMed]
- Gajra A, Newman N, Gamble GP, et al. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol 2003;21:1029-34. [Crossref] [PubMed]
- Amer K, Khan AZ, Parshad R, et al. How can the thoracic surgeon avoid injury to the recurrent laryngeal nerves. Asvide 2018;5:570. Available online: http://www.asvide.com/article/view/25399
- Laccourreye O, Malinvaud D, Delas B, et al. Early unilateral laryngeal paralysis after pulmonary resection with mediastinal dissection for cancer. Ann Thorac Surg 2010;90:1075-8. [Crossref] [PubMed]
- Pisanu A, Porceddu G, Podda M, et al. Systematic review with meta-analysis of studies comparing intraoperative neuromonitoring of recurrent laryngeal nerves versus visualization alone during thyroidectomy. J Surg Res 2014;188:152-61. [Crossref] [PubMed]
- Amer K, Khan AZ, Parshad R, et al. VATS across mediastinal midline nodal harvesting. Asvide 2018;5:571. Available online: http://www.asvide.com/article/view/25400
- Zhang W, Wei Y, Jiang H, et al. Thoracotomy is better than thoracoscopic lobectomy in the lymph node dissection of lung cancer: a systematic review and meta-analysis. World J Surg Oncol 2016;14:290. [Crossref] [PubMed]
- Licht PB, Jørgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96:943-9; discussion 949-50. [Crossref] [PubMed]
- Watanabe A, Koyanagi T, Ohsawa H, et al. Systematic node dissection by VATS is not inferior to that through an open thoracotomy: A comparative clinicopathologic retrospective study. Surgery 2005;138:510-7. [Crossref] [PubMed]
- Palade E, Passlick B, Osei-Agyemang T, et al. Video-assisted vs open mediastinal lymphadenectomy for Stage I non-small-cell lung cancer: results of a prospective randomized trial. Eur J Cardiothorac Surg 2013;44:244-9. [Crossref] [PubMed]
- Osarogiagbon RU, Yu X. Comparative survival of resected node-negative non-small cell lung cancer with and without lymph node examination in the SEER database. J Thorac Oncol 2012;7:abstr 131.
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association (ANITA)): a randomised controlled trial Lancet Oncol 2006;7:719-27. [Crossref] [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351-60. [Crossref] [PubMed]
- Tsuboi M, Ohira T, Saji H, et al. The present status of postoperative adjuvant chemotherapy for completely resected non-small cell lung cancer. Ann Thorac Cardiovasc Surg 2007;13:73-7. [PubMed]
- Pisters KM, Vallières E, Crowley JJ, et al. Surgery with or without preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol 2010;28:1843-9. [Crossref] [PubMed]
- Naur TMH, Konge L, Clementsen PF. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Staging of Patients with Non-Small Cell Lung Cancer without Mediastinal Involvement at Positron Emission Tomography-Computed Tomography. Respiration 2017;94:279-84. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Lim E, McElnay PJ, Rocco G, et al. Invasive mediastinal staging is irrelevant for PET/CT positive N2 lung cancer if the primary tumour and ipsilateral lymph nodes are resectable. Lancet Respir Med 2015;3:e32-e33. [Crossref] [PubMed]
- Lim E, Harris G, Patel A, et al. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer: systematic review and indirect comparison meta-analysis of randomized trials. J Thorac Oncol 2009;4:1380-8. [Crossref] [PubMed]
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65:iii1-27. [Crossref] [PubMed]
- National Institute of Clinical Excellence. The diagnosis and treatment of lung cancer (update), 2011. Available online: https://www.nice.org.uk/guidance/cg121 (last accessed 29 April 2018).
- Robinson LA, Ruckdeschel JC, Wagner H Jr, et al. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:243S-265S.
- Stamatis G, Fechner S, Hillejan L, et al. Repeat mediastinoscopy as a restaging procedure. Pneumologie 2005;59:862-6. [Crossref] [PubMed]
- Pauwels M, Van Schil P, De Backer W, et al. Repeat mediastinoscopy in the staging of lung cancer. Eur J Cardiothorac Surg 1998;14:271-3. [Crossref] [PubMed]
- Amer K, Khan AZ, Parshad R, et al. VATS nodal dissection: caveats, complications and troubleshooting. Asvide 2018;5:572. Available online: http://www.asvide.com/article/view/25401
- Lopez D, Asher CR. Congenital Absence of the Pericardium. Prog Cardiovasc Dis 2017;59:398-406. [Crossref] [PubMed]
Cite this article as: Amer K, Khan AZ, Parshad R, Jones A. VATS lymph node dissection and staging: the Southampton experience. Video-assist Thorac Surg 2018;3:25.