
Uniportal video-assisted thoracoscopic surgery (VATS) for ground-glass opacity (GGO)
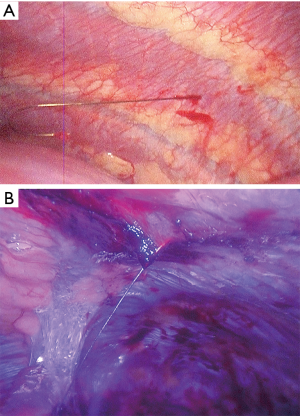
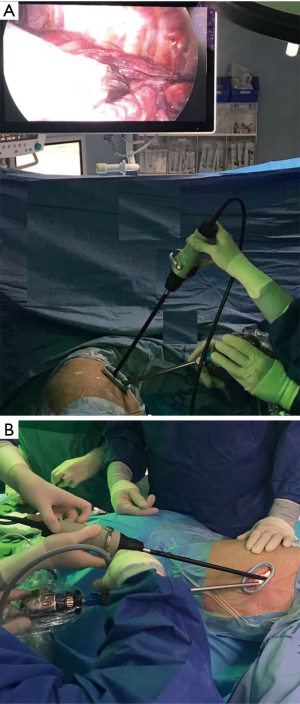
Ground glass opacity “GGO” lesions described radiologically as a focal or diffuse veil-like slightly increased computed tomography (CT) attenuation opacification of the lung through normal lung parenchyma structures. These lesions do not obscure the vascular structures or the rest of the anatomic details and do not yield air-bronchograms (1-3). It indicates sub-threshold parenchymal abnormalities below the spatial resolution of high-resolution CT (HRCT) and has attenuation between 300 and 600 HU (1,2). It is clear that not every GGO lesion is attributed to a malignant or pre-malignant pathology. Many other diseases of the lungs, such as acute or chronic infections and inflammatory pathologies, may come in the form of GGO (4). However, these diseases may be outside the scope of this article. The increased use of CT scan in daily practice and the adoption of the screening systems for lung cancer by some countries, such as China and the United States led to increasing the incidence of GGO lesions and to an increase on the number of patients undergoing surgeries for GGO resections (3). Most of GGOs (37% to 70% of cases) fade or disappear during follow-up with or without antibiotics therapy, and lesions that increase in size or petrify during the follow-up period must be taken seriously as the probability of containing the malignant cells may be higher (4,5). Adenocarcinoma accounts for 40% of lung cancer (6). After the term bronchoalveolar carcinoma “BAC” was a common term used, this type of lung cancer has been newly divided into five main subtypes: Atypical adenomatous hyperplasia “AAH” and Adenocarcinoma in situ “AIS” (these two are the pre-invasive types), Minimally invasive adenocarcinoma “MIS”, invasive adenocarcinoma, and variants of invasive adenocarcinoma (7). It is noticeable that there are differences in morphology and prognosis among the five species mentioned above (7). Since the eventuality of nodal metastases is much lower in GGOs (8), recent attention has focused on anatomical or non-anatomical sub-lobar resections may be an alternative to standard lobectomy (9,10). As mentioned before, GGO lesions that do not disappear during the follow-up period are often malignant or pre-malignant (11). According to recent retrospective studies, it appears that the resection of these lesions is very effective with a very low recurrence rate which is close to zero (12,13). Not so long ago when thoracotomy was the most common way to access to the lung and the thoracic cavity, it was not an easy for the pulmonologist or for the surgeon and of course for the patient to accept the idea of opening the chest in order to resect a small tiny GGO. Everyone was inclined to watch and follow up as much as possible. Surgery was often a last resort and was not seldom to come late. With the development of thoracoscopic surgery, and after the uniportal VATS became a routinely conducted surgeries in many centers around the world, the trend toward eradicating these lesions has become more liberal and easier to accept by all. No doubt that VATS surgeries in general and the recent developments that reduced the invasiveness of operations more and more with the uniportal and subxiphoid approaches, began to change the rules of the game. As surgeons gain greater experience in this field, GGOs can be treated with more rigorous, faster and safer surgeries. Despite all the above and regardless of all the advantages of the VATS, there are still some obstacles that face us. Perhaps the most important hitch is to identify the location of the lesion. This can easily be applied with digital palpation through a thoracotomy. However, this may be somewhat limited in VATS, especially when the tumor in an area that is far from the incision. Distance between the nodule and the pleural surface is the most important determinate of thoracoscopic visibility (14). Nodules deep to the pleural surface must be localized preoperatively. There are many solutions to this problem, and each has advantages and disadvantages. With the beginning of the 1990s, with the development of VATS, publications began to appear to describe ways for locating pulmonary nodules. Wicky et al. described a series of CT-guided localization of pulmonary nodules with methylene blue injection. Good results were shown throughout this paper. In addition to the lack of surgeon radiation, the technique was proven to be safe and effective to avoid thoracotomy in most of cases, but disadvantage with this technique was the spread of methylene blue on the lung surface away from the site of injection, which may force the surgeon to resect excessive pulmonary tissue (15). To prevent this happening, there have been attempts to mix the dye with other materials that may limit its spread over the surface such as cyanoacrylate adhesive, collagen or autologous blood (16-18). These binding materials have improved the success of localization, but sometimes they may be difficult to mix, some of which may cause a negative reaction. Minor complications as pneumothorax and intra-parenchymal bleeding were reported after puncturing the lung. Although rare, but serious complication as anaphylaxis was also reported (19). Hook wire marking was derived from breast operations, where the same wire used in marking the breast lesions was initially used. This method also provides the lack of exposure of the surgeon to the harmful radiations and the abandonment of the fluoroscope during the procedure in addition to the high success rates, which exceed 95% according to most studies (20-23). However, pneumothorax and dislodgment of the wire are still obstacles in this method. Pneumothorax incidence after hook wire insertion in variable in the literature (8–40%) (21-24) and its usually subclinical. Dislodgment (Figure 1) was reported in about 5% of cases in most publications and usually occurs during the manipulation of the lung (especially when there are dense lung adhesions) or at the time of lung deflation (21-24). Significant complications such as massive hemothorax and air embolism were rarely reported. Microcoil and fiducial marking is a technique that is somewhat similar to the hook wire placement with similar success rates and complications but is more comfortable to the patient. The microcoil is placed and deployed to the lung via a coaxial needle, with no wire protruding outside the patient’s body while waiting the surgery. However, this technique requires the use of fluoroscopy, which may consume additional time of the procedure, in addition to exposing the patient and medical staff to radiation (25-27). Some have taken the idea of injecting the lipidol or barium under the guidance of CT in order to localize the lesions. This technique requires the use of fluoroscopy during the surgery, in addition to the fact that the barium may be harmful to the lung and may affect the accuracy of pathological diagnosis. That’s the reason why lipidol is preferred (28,29). Other advantages to lipidol are its durability, and its slow diffusion. To increase the chances of success and accuracy, there are those who experimented guided localization in hybrid operating room (30) and others reported the utilization of dual techniques such as injecting lipidol at the same time with the hook wire insertion which resulted in excellent results (31). Intraoperative near-infrared imaging (NIR) is a newly introduced technique that relies on the injection of indocyanine green. Using the NIR imaging device, hidden tumors could be detected but this technique carries higher false positive and false-negative rates than other methods (32). Intraoperative ultrasound has been used during the past, but in a limited tendency. Although it is an effective method to localize GGOs with excellent success rate, its limitation is the need of a radiologist with experience in ultrasound, which is difficult to provide constantly. But this way is still promising, especially with the great development which occurs on ultrasound devices and the possibility of teaching surgeons to use this tool (33-36). The basic difference between the conventional multi-portal VATS and the uni-portal technique is the instrumentation and the space availability within the incision during the operation through which the instruments are introduced. Since most tumors localization methods are applied prior to the surgery, this has no relevance to the number of ports that will be used. The use of intraoperative ultrasound may be a different situation because of the need to insert the probe (Figure 2) through the wound may be maladjusted with instruments if not done the right way. Probably the best way to avoid this is to insert the probe in the posterior part of the incision while keeping the thoracoscope at the anterior part of it and then put the rest of the instruments in between thus the ultrasound could be used smoothly and effectively (Figures 3,4). Finally, all of the above-mentioned methods do not at all replace the thorough broad familiarity of the surgeon with the surgical anatomy of the lungs and their segments. In cases where tumors marking methods are not available, surgeon should able to know how to determine the location of the lesion and perform the resection according to the segment located in it.

Conclusions
GGOs lesions may be benign, malignant or pre-malignant. Due to the increasing availability of CT scan and the development of screening systems, we see this kind of lesions more often. With the development of minimally invasive techniques, surgeons tend to be more liberal for resecting GGO lesions. In order to overcome the obstacle of locating the tumor, there are many useful ways, each of which has advantages and disadvantages. No doubt the meticulous surgeon’s knowledge of the details of thoracic anatomy is indispensable for this type of operations.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci and Roberto Crisci) for the series “VATS Special Issue dedicated to the 4th international VATS Symposium 2017” published in Video-Assisted Thoracic Surgery. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.04.03). The series “VATS Special Issue dedicated to the 4th international VATS Symposium 2017” was commissioned by the editorial office without any funding or sponsorship. DGR serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Jul 2016 to May 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Remy-Jardin M, Remy J, Giraud F, et al. Computed tomography assessment of ground-glass opacity: semiology and significance. J Thorac Imaging 1993;8:249-64. [Crossref] [PubMed]
- Collins J, Stern EJ. Ground-glass opacity at CT: the ABCs. AJR Am J Roentgenol 1997;169:355-67. [Crossref] [PubMed]
- Pedersen JH, Saghir Z, Wille MM, et al. Ground-Glass Opacity Lung Nodules in the Era of Lung Cancer CT Screening: Radiology,Pathology, and Clinical Management. Oncology (Williston Park) 2016;30:266-74. [PubMed]
- Miller WT Jr, Shah RM. Isolated diffuse ground-glass opacity in thoracic CT: causes and clinical presentations. AJR Am J Roentgenol 2005;184:613-22. [Crossref] [PubMed]
- Godoy MC, Naidich DP. Overview and strategic management of subsolid pulmonary nodules. J Thorac Imaging 2012;27:240-8. [Crossref] [PubMed]
- SEER cancer statistics review, 1975–2008. Available online: http://seer.cancer.gov/csr/1975_2008/results_merged/sect_15_lung_bronchus.pdf
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidis- ciplinary classification of lung adencarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Suzuki K, Kusumoto M, Watanabe S, et al. Radiologic classification of small adenocarcinoma of the lung: radiologic- pathologic correlation and its prognostic impact. Ann Thorac Surg 2006;81:413-9. [Crossref] [PubMed]
- Kohno T, Fujimori S, Kishi K, et al. Safe and effective minimally invasive approaches for small ground glass opacity. Ann Thorac Surg 2010;89:S2114-7. [Crossref] [PubMed]
- Mun M, Kohno T. Efficacy of thoracoscopic resection for multifocal bronchioloalveolar carcinoma showing pure ground- glass opacities of 20 mm or less in diameter. J Thorac Cardiovasc Surg 2007;134:877-82. [Crossref] [PubMed]
- Takashima S, Maruyama Y, Hasegawa M, et al. CT findings and progression of small peripheral lung neoplasms having a replacement growth pattern. AJR Am J Roentgenol 2003;180:817-26. [Crossref] [PubMed]
- Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005;129:991-6. [Crossref] [PubMed]
- Yamada S, Kohno T. Video-assisted thoracic surgery for pure ground-glass opacities 2 cm or less in diameter. Ann Thorac Surg 2004;77:1911-5. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: Indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Wicky S, Mayor B, Cuttat JF, et al. CT-guided localizations of pulmonary nodules with methylene blue injections for thoracoscopic resections. Chest 1994;106:1326-8. [Crossref] [PubMed]
- Yoshida J, Nagai K, Nishimura M, et al. Computed tomography-fluoroscopy guided injection of cyanoacrylate to mark a pulmonary nodule for thoracoscopic resection. Jpn J Thorac Cardiovasc Surg 1999;47:210-3. [Crossref] [PubMed]
- McCormack PM, Ginsberg KB, Bains MS, et al. Accuracy of lung imaging in metastases with implications for the role of thoracoscopy. Ann Thorac Surg 1993;56:863-5. [Crossref] [PubMed]
- McConnell PI, Feola GP, Meyers RL. Methylene blue-stained autologous blood for needle localization and thoracoscopic resection of deep pulmonary nodules. J Pediatr Surg 2002;37:1729-31. [Crossref] [PubMed]
- Wu TT, Chang YC, Lee JM, et al. Anaphylactic reaction to patent blue V used in preoperative computed tomography-guided dye localization of small lung nodules. J Formos Med Assoc 2016;115:288-9. [Crossref] [PubMed]
- Mack MJ, Gordon MJ, Postma TW, et al. Percutaneous localization of pulmonary nodules for thoracoscopic lung resection. Ann Thorac Surg 1992;53:1123-4. [Crossref] [PubMed]
- Eichfeld U, Dietrich A, Ott R, et al. Video-assisted thoracoscopic surgery for pulmonary nodules after computed tomography-guided marking with a spiral wire. Ann Thorac Surg 2005;79:313-6; discussion 316-7. [Crossref] [PubMed]
- Dendo S, Kanazawa S, Ando A, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology 2002;225:511-8. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Chen YR, Yeow KM, Lee JY, et al. CT-guided hook wire localization of subpleural lung lesions for video-assisted thoracoscopic surgery (VATS). J Formos Med Assoc 2007;106:911-8. [Crossref] [PubMed]
- Mayo JR, Clifton JC, Powell TI, et al. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology 2009;250:576-85. [Crossref] [PubMed]
- Sancheti MS, Lee R, Ahmed SU, et al. Percutaneous fiducial localization for thoracoscopic wedge resection of small pulmonary nodules. Ann Thorac Surg 2014;97:1914-8; discussion 1919.
- Toba H, Kondo K, Miyoshi T, et al. Fluoroscopy-assisted thoracoscopic resection after computed tomography-guided bronchoscopic metallic coil marking for small peripheral pulmonary lesions. Eur J Cardiothorac Surg 2013;44:e126-32. [Crossref] [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Moon SW, Wang YP, Jo KH, et al. Fluoroscopy-aided thoracoscopic resection of pulmonary nodule localized with contrast media. Ann Thorac Surg 1999;68:1815-20. [Crossref] [PubMed]
- Zhao ZR, Lau RW, Ng CS. Hybrid theatre and alternative localization techniques in conventional and single-port video-assisted thoracoscopic surgery. J Thorac Dis 2016;8:S319-27. [PubMed]
- Kang DY, Kim HK, Kim YK, et al. Needlescopy-assisted resection of pulmonary nodule after dual localisation. Eur Respir J 2011;37:13-7. [Crossref] [PubMed]
- Keating JJ, Kennedy GT, Singhal S. Identification of a subcentimeter pulmonary adenocarcinoma using intraoperative near-infrared imaging during video-assisted thoracoscopic surgery. J Thorac Cardiovasc Surg 2015;149:e51-3. [Crossref] [PubMed]
- Piolanti M, Coppola F, Papa S, et al. Ultrasonographic localization of occult pulmonary nodules during video-assisted thoracic surgery. Eur Radiol 2003;13:2358-64. [Crossref] [PubMed]
- Abu Akar F, Gonzalez-Rivas D, Ismail M, et al. Uniportal video-assisted thoracic surgery: the Middle East experience. J Thorac Dis 2017;9:871-7. [Crossref] [PubMed]
- Wada H, Anayama T, Hirohashi K, et al. Thoracoscopic ultrasonography for localization of subcentimetre lung nodules. Eur J Cardiothorac Surg 2016;49:690-7. [Crossref] [PubMed]
- Abu Akar F, Gonzalez-Rivas D, Ismail M, et al. Using Intra-operative Ultrasonography for finding small lung lesions. Asvide 2017;4:064. Available online: http://www.asvide.com/articles/1363
- Abu Akar F, Gonzalez-Rivas D, AbdelRahman N, et al. Technique of ultrasound-guided localization of lung nodule. Asvide 2018;5:509. Available online: http://www.asvide.com/article/view/24683
Cite this article as: Abu Akar F, Gonzalez-Rivas D, AbdelRahman N, Zhu Y. Uniportal video-assisted thoracoscopic surgery (VATS) for ground-glass opacity (GGO). Video-assist Thorac Surg 2018;3:19.



