
Thoracoscopic (video-assisted thoracoscopic surgery) pneumonectomy, technical details and literature review
Introduction
Minimally invasive approaches to lobar and sublobar resection are now widely accepted to be beneficial when compared to thoracotomy. Since its first description over 20 years ago, widespread adoption of video-assisted thoracoscopic surgery (VATS) approaches for lobectomy was slowed by concerns largely based largely on safety and oncologic validity. These have largely been disproven, and now over 60% of lobectomies being performed as documented with the STS database are done by VATS (1-6). Most previous exclusion criteria for approaching patients by VATS have been overcome safely (7).
Obstacles to the widespread adoption of VATS for pneumonectomy are unique compared to those associated with the VATS lobectomy learning curve. Large, bulky tumor pathology often involving the hilum, inability to gain control of catastrophic vascular injury at the level of the main pulmonary artery, and the known morbidity and mortality associated with pneumonectomy in general all contribute to the relatively small level of experience reported for VATS pneumonectomy. As a result, approaching whole lung resection by VATS has understandably been less common. Reports are largely limited to case reports, small case series, and single institution experiences (8-22).
Existing evidence regarding the potential advantages for VATS pneumonectomy over standard thoracotomy is lacking, but it is not unreasonable to assume that many of the advantages realized for VATS lobectomy would translate to those operations requiring more extensive lung resection if oncologic principles are maintained. As surgeon experience has increased simultaneously with improved surgical instrumentation and videoscopic technology, approaching pneumonectomy by VATS has become feasible. Here we review the pertinent technical aspects associated with performing VATS pneumonectomy, as well as review recent literature associated with it.
Technical considerations for VATS pneumonectomy
Preoperative preparation
Preoperative evaluation for thoracoscopic pneumonectomy does not differ than that for open pneumonectomy and consists of a standard cardiopulmonary work up including transthoracic echocardiogram and pulmonary function testing. For patients with marginal cardiopulmonary function, routine split lung function testing and cardiopulmonary exercise testing are obtained. In general whole lung resection is avoided when possible, and every effort is made to perform sleeve resection to spare lung function given the significant morbidity and mortality rates associated with pneumonectomy (23). Frailty testing is conducted on patients over 75 years of age, which may uncover surgical risks not apparent on standard cardiac and pulmonary testing. Planning for intraoperative transesophageal echocardiogram (TEE) can be useful in assessing cardiac function at the time of pulmonary artery clamping. When there are conflicting or borderline predictive data, a right sided cardiac catheterization is considered. Despite its invasiveness, the ipsilateral pulmonary artery can be balloon-occluded while stimulating the cardiac output, measuring right ventricular pressure response and assessing systemic arterial oxygen saturations.
Operative considerations
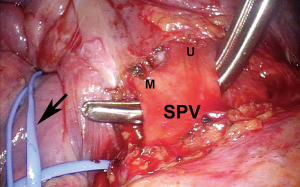
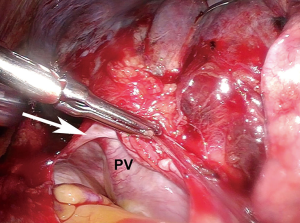
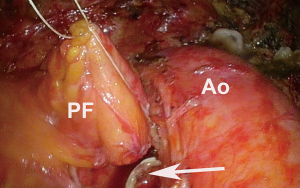
After ensuring the patient has adequate cardiopulmonary reserve, the important anatomic considerations for preoperative surgical planning for VATS pneumonectomy are largely centered around the isolation and division of four hilar structures. The pulmonary veins are routinely approached first. Instead of dividing each vein immediately after it has been dissected free, both are dissected out first unless one requires division to improve exposure of the other. By dissecting both before dividing either one, they can then be divided in rapid succession so that attention can be turned towards isolating the main left pulmonary artery without delay (Figure 1). This minimizes the vascular congestion that may occur from systemic bronchial artery collateral circulation in the lung while time is spent isolating and dividing the pulmonary artery. For completion pneumonectomy cases, vein division may also accelerate blood losses from denuded lung parenchyma. Often, due to the effects of induction therapy or the location of the tumor at the hilum, the vein dissection is carried out more safely within the pericardial cavity (Figure 2).
When surgical staging of the mediastinum is to be performed, it is done at the same operative setting as the planned lung resection, and can be helpful in making the hilar dissection safer and easier. During video mediastinoscopy, the dissection is carried onto the mainstem bronchus, thus starting the separation of the pulmonary artery from the bronchus. This step facilitates an easier and potentially safer dissection of the main pulmonary artery from the bronchus within the chest.
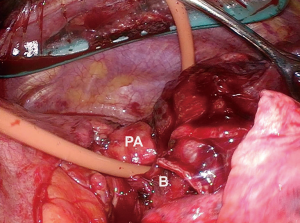
When dissecting the main pulmonary artery from the mainstem bronchus, care must be taken to dissect towards the bronchus with blunt dissection to avoid potential injury to the artery. When this is achieved, a red rubber catheter can be placed in between the two structures and used as a guide to safely bring a stapler across the artery (Figure 3). To facilitate safe passage of the stapler, care must be taken after passage of the red rubber catheter to dissect off any additional peribronchial or adventitial tissue that may serve as an impediment. Prior to firing the stapler, it is closed and the patient is monitored for any hemodynamic compromise that may suggest compromise of the main pulmonary artery.
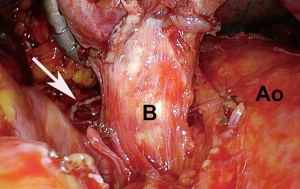
Once the pulmonary artery has been divided, the bronchus is dissected up to the level of the carina to avoid a long bronchial stump just as with an open resection. Manipulating the specimen when dissecting the bronchus is aided with the use of a 5-mm laparoscopic flexible liver retractor such as the Diamond-Flex (CareFusion, San Diego, CA, USA). It allows for upward traction for safe and thorough dissection of the mainstem bronchus, keeping the lung, and potentially large tumors out of the way (Figure 4).
Coverage for the bronchial stump is performed routinely to minimize the dreaded complication of bronchopleural fistula. This can be achieved with various methods, including a rotational pleural flap, pericardial fat pad, intercostal muscle flaps, as well as the azygos vein on the right (Figure 5).
Right pneumonectomy
As with open right pneumonectomy, risk for postoperative respiratory failure is significant, but from a purely technical standpoint can be less challenging due to easier exposure of the right main pulmonary artery and proximal right mainstem bronchus.
Left pneumonectomy
In contrast to right pneumonectomy, the risk for postoperative respiratory failure with left pneumonectomy is less, though the technical demands for the dissection can be more challenging. The shorter length of the left main pulmonary artery often requires an intra-pericardial dissection to gain proximal control safely.
Discussion
Despite known risks for perioperative morbidity and mortality associated with it, pneumonectomy is sometimes required to ensure an R0 resection of non-small cell lung cancer (NSCLC). While minimally invasive approaches have become routine for sublobar and lobar resections, thoracotomy remains the standard for whole lung resection. Reasons for this likely involve the known physiologic insult to the patient, the potential for catastrophic complications working on hilar structures, and large bulky tumor/lymph node pathology. Increasing surgeon experience with minimally invasive procedures for sublobar and lobar resections has allowed for approaching complex tumor pathology by VATS, including pathology requiring pneumonectomy.
Laboratory experiments with animals have demonstrated potential benefits for thoracoscopic approaches to pneumonectomy. Acute phase reactive proteins measured in dogs undergoing whole lung resection by VATS compared to thoracotomy showed that despite increased operative time, serum levels of C-reactive protein on POD #3 and the WBC count on POD#1 were significantly lower for dogs completed by VATS. In a similar study where pigs underwent pneumonectomy by VATS versus open approaches, C-reactive protein and Il-6 measurements for pigs that underwent VATS were significantly lower for the VATS group on POD#1. Serum cortisol levels for the thoracotomy group were significantly elevated postoperatively compared to those done by VATS. Despite significantly longer operative times for the VATS group, no physiologic differences were noted postoperatively in the two groups (24,25).
Clinical results reported for thoracoscopic pneumonectomy are largely limited to case reports and small case series. The first described video-assisted thoracoscopic pneumonectomy was reported by Walker in 1994 (8). Table 1 lists an additional thirteen case reports or small case series that have been reported (9-22) since then. Single incision VATS pneumonectomy was reported in 2013, and another report exists for an awake non-intubated pneumonectomy (for non-malignant pathology) (16,17). Importantly, to date small case series have not demonstrated a thoracoscopic pneumonectomy to be unsafe. In 2016 Liu retrospectively evaluated 32 patients who underwent VATS pneumonectomy and compared them to 64 patients who underwent conventional thoracotomy. No difference was seen based on approach for transfusion rates, hospital length of stay (LOS), dissected lymph node numbers, dissected lymph node stations, or estimated blood loss. Overall complication rates were similar for both groups at 20.0% and 22.5%. VATS cases did require more operative time (187.5 vs. 146.3 min) (11).
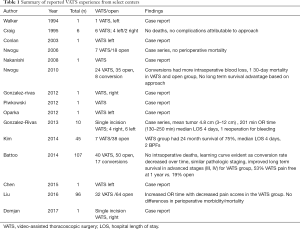
Full table
We have previously reported our single institution experience spanning over 10 years at an NCI designated cancer center. The retrospective review of all patients undergoing pneumonectomy included 101 consecutive cases, of which 64 were attempted by VATS. Conversion from VATS to thoracotomy was required in 17 cases. Preoperative characteristics were similar in the groups except for greater age, female sex, and preoperative comorbidities in the VATS group. Clinical stage was lower in the VATS group, but more upstaging occurred in this group, and median survival for pathologic stage III and IV patients was higher for patients approached by VATS. The percentage of successful completion of VATS pneumonectomy improved from 26% during the first half of the series to 63% during the second half of the series. There were no intraoperative deaths related to technical issues or bleeding.
In summary, approaching pneumonectomy by VATS by experienced surgeons can be a safe strategy that does not appear to compromise oncologic principles. Further investigations are needed to determine potential impact on long term outcomes. When approaching VATS pneumonectomy regardless of the side, the importance of gaining proximal control of the main pulmonary artery must be emphasized. The timing of this maneuver occurs after dissection and division of both pulmonary veins. With control of the main pulmonary artery being the most critical and stress-inducing step, key attention must be paid to keep the dissection close to the bronchial wall, thus minimizing potential for vessel injury.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Video-Assisted Thoracic Surgery for the series “VATS for Locally Advanced Lung Cancer”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.04.02). The series “VATS for Locally Advanced Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. TLD served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Apr 2017 to May 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hartwig MG, D'Amico TA. Thoracoscopic lobectomy: the gold standard for early-stage lung cancer? Ann Thorac Surg 2010;89:S2098-101. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9; discussion 1250. [Crossref] [PubMed]
- Seder CW, Salati M, Kozower BD, et al. Variation in Pulmonary Resection Practices Between The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons General Thoracic Surgery Databases. Ann Thorac Surg 2016;101:2077-84. Erratum in: Ann Thorac Surg 2017;104:2129. [Crossref] [PubMed]
- Yan TD, Cao C, D'Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]
- Walker WS, Carnochan FM, Mattar S. Video-assisted thoracoscopic pneumonectomy. Br J Surg 1994;81:81-2. [Crossref] [PubMed]
- Oparka J, Yan TD, Richards JM, et al. Video-assisted thoracoscopic pneumonectomy: the Edinburgh posterior approach. Ann Cardiothorac Surg 2012;1:105-8. [PubMed]
- Chen HW, Du M. Video-assisted thoracoscopic pneumonectomy. J Thorac Dis 2015;7:764-6. [PubMed]
- Liu Y, Gao Y, Zhang H, et al. Video-assisted versus conventional thoracotomy pneumonectomy: a comparison of perioperative outcomes and short-term measures of convalescence. J Thorac Dis 2016;8:3537-42. [Crossref] [PubMed]
- Nakanishi R, Hirai A, Yamashita T, et al. Video-assisted thoracoscopic completion pneumonectomy for a second primary cancer: a case report. J Thorac Cardiovasc Surg 2008;135:945-6. [Crossref] [PubMed]
- Piwkowski C, Gabryel P, Kasprzyk M, et al. Video-assisted thoracic surgery pneumonectomy: the first case report in Poland. Wideochir Inne Tech Maloinwazyjne 2012;7:197-201. [Crossref] [PubMed]
- Craig SR, Walker WS. Initial experience of video assisted thoracoscopic pneumonectomy. Thorax 1995;50:392-5. [Crossref] [PubMed]
- Conlan AA, Sandor A. Total thoracoscopic pneumonectomy: indications and technical considerations. J Thorac Cardiovasc Surg 2003;126:2083-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Uniportal video-assisted thoracoscopic pneumonectomy. J Thorac Dis 2013;5:S246-52. [PubMed]
- Hung WT, Liao HC, Cheng YJ, et al. Nonintubated Thoracoscopic Pneumonectomy for Bullous Emphysema. Ann Thorac Surg 2016;102:e353-5. [Crossref] [PubMed]
- Kim AW, Fonseca AL, Boffa DJ, et al. Experience with thoracoscopic pneumonectomies at a single institution. Innovations (Phila) 2014;9:82-6; discussion 86. [Crossref] [PubMed]
- Nwogu CE, Yendamuri S, Demmy TL. Does thoracoscopic pneumonectomy for lung cancer affect survival? Ann Thorac Surg 2010;89:S2102-6. [Crossref] [PubMed]
- Nwogu CE, Glinianski M, Demmy TL. Minimally invasive pneumonectomy. Ann Thorac Surg 2006;82:e3-4. [Crossref] [PubMed]
- Battoo A, Jahan A, Yang Z, et al. Thoracoscopic pneumonectomy: an 11-year experience. Chest 2014;146:1300-9. [Crossref] [PubMed]
- Domjan M, Mavko A, Štupnik T. Single-port video-assisted thoracoscopic surgery (VATS) right pneumonectomy: a case report. J Vis Surg 2017;3:130. [Crossref] [PubMed]
- Abdelsattar ZM, Shen KR, Yendamuri S, et al. Outcomes After Sleeve Lung Resections Versus Pneumonectomy in the United States. Ann Thorac Surg 2017;104:1656-64. [Crossref] [PubMed]
- Liu HF, Gao L, Liu T, et al. Comparison of acute phase reaction and postoperative stress in pigs undergoing video-assisted thoracoscopic versus thoracotomy pneumonectomy. Acta Vet Scand 2016;58:75. [Crossref] [PubMed]
- Liu HF, Ren QM, Wang ZB, et al. Comparison of acute phase protein and hemodynamic variables in dogs undergoing video-assisted thoracoscopic vs. open pneumonectomy. Exp Ther Med 2017;13:2391-8. [Crossref] [PubMed]
Cite this article as: Hennon MW, Demmy TL. Thoracoscopic (video-assisted thoracoscopic surgery) pneumonectomy, technical details and literature review. Video-assist Thorac Surg 2018;3:16.


