
Video assisted thoracic surgery with chest wall resection
Introduction
The main indications for chest wall resection include primary tumors of the chest wall, soft tissue, bone or cartilage as well as lung neoplasms with chest wall invasion. This often necessitates removal of ribs, cartilage, and chest wall soft tissue to the extent that a large thoracotomy incision has traditionally used. Furthermore, depending on the size and location of the resultant chest wall defect, chest wall reconstruction is often necessary to maintain thoracic structure, preserve respiratory mechanics, and protect the underlying intrathoracic structures.
Given the large nature of these procedures, it is perhaps not surprising that chest wall resection via thoracotomy has been associated with relatively high postoperative morbidity and mortality. For instance, a recent analysis from the Surveillance Epidemiology and End Results (SEER)-Medicare Database evaluating patients undergoing lung resection for non-small cell lung cancer (NSCLC) found that concomitant chest wall resection was a leading risk factor for early perioperative mortality (1). The increased perioperative risk faced by patients undergoing chest wall resection has largely been attributed to postoperative pain and perturbed respiratory mechanics that result from the large thoracotomy incision, rib-spreading, and alteration of chest wall structure. These factors contribute to increased risk of respiratory insufficiency, which is the principal driver of early postoperative morbidity and mortality in the general thoracic surgery population
To a large degree, patients with chest wall tumors have not benefited from the widespread adoption of video-assisted thoracic surgery (VATS) that has occurred over the last two to three decades. Since the first VATS lung resection in 1992, refinement in technique and improved minimally invasive technology have led VATS to become the preferred approach for thoracic procedures over thoracotomy whenever technically feasible (2-18). VATS lung resections are generally performed through one to three small incisions without any rib spreading. As a result, VATS patients have considerably less post-operative pain and less perturbation of respiratory mechanics. VATS compared with thoracotomy has also been associated with a lower incidence of postoperative atrial fibrillation and numerous other postoperative complications. All this allows for more rapid mobilization of the patient and provides for a shorter length of hospitalization. Moreover, aside from the reduced morbidity imparted by VATS, it has been well established that VATS lung resections for cancer do not compromise oncologic outcomes in comparison to thoracotomy (5,7).
Nevertheless, the advancement of VATS has largely not been transferred to surgery for tumors involving the chest wall. This is the likely the result of the technical challenges brought about by the bony chest wall anatomy, which encumber a minimally invasive approach, coupled with the perception that requisite chest wall resection mitigates any postoperative pain reduction that may be gained with a minimally invasive approach. In fact, chest wall tumor involvement is viewed by the majority of the thoracic surgery community view as a contraindication for a VATS based approach and it is among the most common reasons for intraoperative conversion to thoracotomy (5,19). Still, a number of groups have successfully used VATS techniques for resection of lung tumors with chest wall invasion as well as for resection of primary chest wall tumors and benign chest wall conditions. The experience with such techniques is still limited at present, but the feasibility of these techniques for selected situations has been demonstrated. Furthermore, improving technology will likely continue to facilitate the possibility that lesions involving the chest wall can be addressed through more limited incisions and less tissue trauma. Herein, we review the current status for VATS based techniques for chest wall resection and also review techniques for chest wall reconstruction and the early outcomes associated with these procedures.
Hybrid VATS lung and chest wall resection
Early stage NSCLC with associated chest wall invasion likely represents the most common pathology that might potentially be addressed by VATS based lung and en bloc chest wall resection (20). Other conditions such as metastatic lung neoplasm with chest wall invasion or primary chest wall tumors invading into the lung are less common entities that may also be addressed through such an approach (21,22). With regard to NSCLC, involvement of chest wall structures including the parietal pleura, soft tissue, and boney structures complicates an estimated 5–8% of cases (23,24). While NSCLC chest wall invasion is associated with poorer prognosis than less invasive tumors, if the lesion is otherwise deemed resectable after appropriate clinical staging and neoadjuvant therapy (24,25), then anatomic lung with en bloc chest wall resection has become accepted as the standard to achieve optimal outcomes (24,25). With complete oncologic resection, 5-year survival rates in this population have been pushed upwards of 60%.
As noted, the technical difficulties related to the bony chest wall anatomy as well as reduced parenchymal mobility resulting from lung fixation to the chest wall have hindered progress in the development of minimally invasive techniques for the resection of NSCLC with chest wall invasion. However, given the increased early postoperative complications experienced in this population, there is clearly a need to develop better operative strategies. To that end, several groups have proposed hybrid VATS lung and chest wall resections. While the incisions utilized with these strategies can generally not be limited solely to those used in a traditional VATS lung resection, the basic premise has been to employ a VATS based approach to reduce overall incision size, avoid rib spreading, and limit tissue trauma. In so doing, it is hoped that these strategies in comparison to the traditional open approach will lessen postoperative pain and offer better preservation of thoracic structure and respiratory mechanics, which in turn may improve early postoperative morbidity and mortality.
The general approach for these procedures is an adaptation from the classic VATS lung resection and variations have been described by our group as well as that of Demmy and colleagues (26-29). Following administration of general anesthesia and lung isolation, the patient is placed in the lateral decubitus position with the side of interest up. While it is acceptable for the patient to be positioned precisely lateral (90 degrees), leaning the patient either slightly anterior or posterior may improve access to the tumor via the counter incision. The table itself may be rotated as well to facilitate exposure.
A 5 mm port is placed, most commonly in the 8th intercostal space of the posterior axillary line, and a 4–5 cm utility port is positioned in the anterior axillary line, usually at the 5th intercostal space. Some deviation in the traditional VATS port placement arrangement may be appropriate based on the location of the tumor and associated chest wall invasion. An additional working port may also useful and is ideally situated orthogonal to the site of chest wall invasion to facilitate thoracoscopic instrument manipulation. Once the ports are placed, the lung and pleural space are carefully inspected for the presence of unexpected metastatic disease that would preclude resection. The chest wall is then carefully evaluated to determine the extent of gross invasion and the expected extent of chest wall resection required to ensure the feasibility of resection with a VATS hybrid approach. The decision to then proceed first with either the lung resection portion of the case or the chest wall resection is dependent on case-specific factors or at the surgeon’s preference.
The pulmonary resection is carried out using previously described thoracoscopic techniques, with individual hilar dissection and stapling of the pulmonary arterial and venous branches, stapling of the involved bronchus, and division of the fissure (18). It is usually possible to complete the entire hilar dissection and mediastinal lymph nodes dissection prior to beginning the chest wall resection; however, the chest wall resection itself may facilitate completion of the hilar dissection and may be performed first.
For the chest wall resection, the planned margins for resection are scored circumferentially with electrocautery, exposing the soft tissue elements for resection (Figure 1). Each of the involved ribs should be identified (usually 3), and the anterior aspect of the intended division identified and the periosteum scored with electrocautery. If the tumor is posterior, the posterior margin is usually achieved by division at the costovertebral junction; if the tumor is more anterior, the posterior rib margin is similarly scored. Subsequently, the superior margin and the inferior margin are developed, again using electrocautery, and the intercostal muscle between each rib divided, proximally and distally. Once these margins are delineated, the neurovascular bundles can be ligated with an energy source at the posterior extent of the resection.
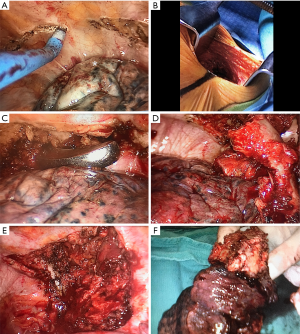
At this point, the location and length of the counter incision is chosen, by passing an instrument above and below the margins of resection into the subcutaneous space and palpating the instrument externally. The counter incision is then made, usually approximately 7 cm, and dissection to the muscular level undertaken. The anterior rib margins are divided with a rib cutter and the specimen is pushed into the chest manually to better expose the posterior margin. For posterior tumors, the involved costovertebral joints are divided with an osteotome, as would be done with an open procedure. Blood loss is minimized due to previous division of the neurovascular bundles. The specimen is then removed via the counter incision. Chest wall reconstruction may then be performed if needed (discussed below).
Superior sulcus tumors (Pancoast tumors), or tumors in the apex of the lung with invasion of the apical chest wall, are especially challenging due to the proximity, and sometimes involvement, of the brachial plexus and subclavian vessels (20). Moreover, the lack of mobility for superior sulcus tumors stemming from chest wall involvement as well as employment of neoadjuvant chemoradiation often causes obscured visualization of the thoracic outlet. Despite these challenges, VATS hybrid approaches have been described both anterior (30-33) and posterior (34) superior sulcus tumors. In their report of a resection of a posterior superior sulcus tumor, Rosso and colleagues used a hybrid VATS technique similar to that described above (34). To allow resection of chest wall structures in the posterior-lateral location, a limited paravertebral counter incision (Shaw-Paulson) was made along the scapula border. Rosso and colleagues have also described a VATS based resection of an anterior superior sulcus tumor using a transmanubrial approach for en bloc chest wall resection (30). Specifically, with the patient in the supine position a 1 cm camera port was placed in the mid-axillary line 8th intercostal space followed by a 4 cm utility port in the anterior axially line at the 4th intercostal space and an additional 1 cm working port more posteriorly. Following hilar dissection and lung resection using standard VATS techniques (5), a C-shaped counter incision was made staring at the anterior border of the sternocleidomastoid and extending the inferiorly to the 2nd intercostal space where it was then extended laterally for 5–6 cm on the affected side. A transmanubrial incision was made in order to preserve the sternoclavicular joint and extended to the second intercostal space. Following soft tissue dissection laterally to obtain a sufficient margin, the 1st and 2nd ribs were divided. The freed specimen and removed through the para-manubrial incision. The resultant anterior chest wall defect was reconstructed by placement of a prosthetic patch.
With the limited reported experience of hybrid VATS lung and en bloc chest wall resection to date, it remains far from a standard procedure. This procedure should be carefully selected on the basis of case-specific anatomic and patient factors and likely should only be pursued by high volume, experienced VATS surgeons. Large tumors (>7 cm) likely will not be amenable to a VATS based approach and the presence of extensive hilar lymphadenopathy or preoperative radiotherapy, which limit hilar mobility, may also not be suitable for minimally invasive techniques. At present, tumor involvement of spinal structures including transverse processes or vertebral bodies is generally considered a contraindication for a minimally invasive approach. However, with improving technology in realm of minimally invasive spinal surgery, it is possible that the development of such techniques may be employed to allow VATS based resections of tumors with boney spine involvement in the future (27). Certainly, for oncologic resections, the hybrid VATS approach should not be employed in patients who would require significant deviation from the traditional VATS port arrangement or surgical strategy on the basis of chest wall involvement that may compromise the ability for anatomic resection.
Primary chest wall tumors
Primary chest wall tumors are a heterogeneous group of neoplasms that arise from the chest wall bone, soft tissue, or cartilage. These tumors occur infrequently, carrying a lifetime incidence of <2% and representing only about 5% of all thoracic neoplasms. The clinical features and management of the most common benign and malignant primary chest wall tumors have been reviewed previously and will not be discussed in depth here (21).
Once diagnosis has been confirmed, the principal treatment of the large majority of chest wall tumors is resection. Exceptions to this include lymphomas, solitary plasmacytoma, and the Ewing family of tumors, which are primarily managed medically. For most malignant tumor histologic subtypes, a 4 cm margin is sought and a rib above and below the center of the tumor should be removed when rib involvement is present. For tumors with high grade malignant histology and bony structure involvement, the entirety of that bony structure (e.g., rib, sternum, manubrium) should be resected along with any involved adjacent structures including, for example, the lung or the pericardium. Given the wide oncologic resection required for management of most malignant primary chest wall tumors, many of these lesions will not be well suited for a minimally invasive approach. However, in selected cases for relatively small tumors, a VATS based approach may allow resection of the lesion while offering better preservation of the chest wall structure and potentially limiting postoperative pain and morbidity. Certainly, for benign chest wall lesions these potential advantages of may be realized without concern of inadequate oncologic resection. This holds true also for other benign thoracic conditions that may require chest wall resection, one of the more common being first rib resection for thoracic outlet syndrome.
In comparison to VATS based resection of primary lung tumors invading the chest wall, there is greater flexibility in port arrangement for the resection for a primary chest wall lesion since anatomic lung resection is not required. Ports may be placed to allow triangulation and optimal access to the area of the interest on the chest wall. The starting point of resection may be initiated with division of ribs and associated soft tissues either thoracoscopically as described above or through limited counter incisions. The rib block can then be retracted away from the overlying chest wall while it is separated from the associated intercostal and overlying soft tissues with an energy sealing device and other dissecting instruments. The finishing point of the resection can then be divided via an intrathoracic approach if feasible or with a limited counter incision. The specimen can then be delivered through an accommodating utility or counter incision followed by chest wall reconstruction if necessary.
To date, descriptions of VATS based resections of primary chest wall tumors and other benign thoracic conditions in the literature are limited to only a few. VATS based strategies have most commonly be described for resection of localized rib tumors, including chondrosarcoma (35), Ewing’s Sarcoma (36), Giant cell tumor (37), and fibrous dysplasia (38). In all cases, a satisfactory resection margin was achieved with a perceived benefit of good postoperative pain control and rapid recovery. We have also described a VATS based technique for 1st rib resection for the management of thoracic outlet syndrome, in which a limited 5–6 cm transaxillary incision is utilized to allow exposure and removal of the first rib under thoracoscopic guidance (39). In total, while the experience remains nascent, growing VATS capability and advancing technologies may possibly expand utilization of minimally invasive approaches for resection of primary chest wall tumors and benign chest wall conditions going forward.
Reconstruction options
Chest wall reconstruction should generally be considered for defects >5 cm or including ≥4 ribs, although there is no consensus on the absolute indications for reconstruction (40). Furthermore, the need for reconstruction is often influenced by case-specific anatomic and physiologic considerations. For example, the need for reconstruction in the apical-posterior portion of the chest wall is often not necessary because of the structural support provided by the scapula and shoulder girdle. In contrast, the anterolateral chest wall has less structural support and resection of this portion of the chest wall causes greater detriment to pulmonary mechanics and is therefore more likely to require reconstruction. The underlying goal of any reconstruction strategy is to reestablish structural stability and soft tissue coverage in order to preserve pulmonary mechanics and prevent chest wall hernia while concomitantly protecting the underlying viscera and providing acceptable cosmesis.
There are varied techniques for chest wall reconstruction, which have been reviewed extensively elsewhere (40). However, generally some combination of soft tissue mobilization with placement of reinforcing prosthetic or cryopreserved homo- or allograft patch to recreate thoracic structure and stability is employed with or without an autologous tissue flap to fill dead space or provide additional needed tissue coverage. Theoretically, a major benefit of VATS based approaches for chest wall resection is the enhanced preservation of chest wall soft tissue (26). This is advantageous towards maintaining some degree of chest wall structure and allowing for more targeted, less complex reconstruction strategies.
Adopting techniques commonly employed in laparoscopic repair of abdominal wall hernia, Demmy and colleagues have described a VATS based approach for chest wall reconstruction without further perturbation to overlying chest wall soft tissue than already introduced by the VATS based resection portion of the procedure (27). Specifically, after introduction of a prosthetic patch that has been sized to cover the portion of resected chest wall through the VATS utility incision, a suture passing device (Cater-Thompson Close-Sure System; CooperSurgical, Inc, Trumbull, Conn, USA) is inserted into the thoracic cavity through stab incisions at the boundaries of the intended reconstruction. This device is used to move sutures through the patch at these locations, which are then tied down at the location of the stab incision to secure the patch in place over the resected rib block. Alternatively to using an external suture passing device, a prosthetic patch may be secured with sutures around the edges of the resected portion of chest wall through and intrathoracic approach using a thoracoscopic needle driver as we have described (41).
Aside from these approaches, there are also isolated reports of VATS based rib reconstruction with titanium platting (38) as well as minimally invasive harvest and transposition of the latissimus dorsi for chest wall reconstruction in Poland Syndrome (42). Thus, while minimally invasive approaches towards chest wall reconstruction remain rare, it is likely that surgeons will continue to push such strategies forward as minimally invasive techniques continue to advance. So long as the principles of chest wall reconstruction are adhered to, there stands a chance for patient benefit when these strategies are coupled with VATS based approaches for chest wall resection.
Outcomes and future directions
Outcomes data relating to VATS based chest wall resection remains limited to a few small case series and case reports at this time. Probably as a result of the technical challenges associated with these procedures as well as the relatively rarity of patients who may be candidates, VATS based chest wall resections are rarely performed and consequently it is difficult to rigorously assess any benefit this approach may provide in comparison to the traditional thoracotomy approach. Still, in the largest series of hybrid VATS lung and chest wall resection, both Berry and colleagues (n=12) (29) and Hennon and colleagues (n=15) (26) were able to demonstrate sufficient oncologic resection among hybrid VATS patients. While both studies were retrospective in nature and consisted of highly selected cohorts, early postoperative morbidity and mortality among hybrid VATS patients was comparable to contemporary and largely similar cohorts managed via thoracotomy. Additionally, hybrid VATS patients had a shorter length of hospitalization, suggesting more rapid convalescence. Both studies also demonstrated a lower incidence in the need for chest wall reconstruction among VATS resection patients (although this finding was not statistically significant in the Berry study), lending weight to the notion that the VATS based chest wall resection offers better preservation of chest wall structure and lessens the need for reconstructive measures in comparison to thoracotomy. Finally, in both these series as well as the collection of case reports describing VATS based chest wall resections, there has been an appreciation of reduced postoperative pain, even if not rigorously proven. As improved pain control and respiratory mechanics have been attributed to improved morbidity in the VATS versus open lobectomy population (10), it is possible that similar benefits may be gained among patients with chest wall involvement by hybrid VATS resection even if these benefits have not yet been demonstrable among the small amount of data available on these patients.
In the future, it can be anticipated that surgeons will continue to modify and explore techniques for a less morbid approach to resection of primary and lung neoplasms with chest wall lesions. With widespread training and growing comfort in VATS techniques (5), we may observe expanded utilization of minimally invasive strategies for chest wall resection, particularly as the feasibility of this approach has been demonstrated without apparent detriment to safety among selected patients. Improving minimally invasive technology may also facilitate this change. Perhaps of special relevance to chest wall associated tumors is the increasing presence of robot assisted thoracic surgery (43). In comparison to VATS instrumentation, the enhanced articulation and dexterity provided with robot assisted surgery may allow chest wall resection to be performed through limited incisions more readily and in a broader population of patients with chest wall lesions. In any event, it will be important to adhere to basic surgical principles relating to chest wall resection and reconstruction as the effort to improve and refine these procedures continues.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Todd Demmy) for the series “VATS for Locally Advanced Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.03.07). The series “VATS for Locally Advanced Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. TAD serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Jul 2016 to May 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hu Y, McMurry TL, Wells KM, et al. Postoperative mortality is an inadequate quality indicator for lung cancer resection. Ann Thorac Surg 2014;97:973-9; discussion 8-9. [Crossref] [PubMed]
- Gaudet MA, D'Amico TA. Thoracoscopic Lobectomy for Non-small Cell Lung Cancer. Surg Oncol Clin N Am 2016;25:503-13. [Crossref] [PubMed]
- Nwogu CE, D'Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015;99:399-405. [Crossref] [PubMed]
- Klapper J, D'Amico TA. VATS versus open surgery for lung cancer resection: moving toward a minimally invasive approach. J Natl Compr Canc Netw 2015;13:162-4. [Crossref] [PubMed]
- Yan TD, Cao C, D'Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]
- Shah RD, D'Amico TA. Modern impact of video assisted thoracic surgery. J Thorac Dis 2014;6:S631-6. [PubMed]
- Berry MF, D'Amico TA, Onaitis MW, et al. Thoracoscopic approach to lobectomy for lung cancer does not compromise oncologic efficacy. Ann Thorac Surg 2014;98:197-202. [Crossref] [PubMed]
- Hennon MW, Demmy TL. Video-assisted thoracoscopic surgery (VATS) for locally advanced lung cancer. Ann Cardiothorac Surg 2012;1:37-42. [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Nwogu CE, Yendamuri S, Demmy TL. Does thoracoscopic pneumonectomy for lung cancer affect survival? Ann Thorac Surg 2010;89:S2102-6. [Crossref] [PubMed]
- Hartwig MG, D'Amico TA. Thoracoscopic lobectomy: the gold standard for early-stage lung cancer? Ann Thorac Surg 2010;89:S2098-101. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- D'Amico TA. Long-term outcomes of thoracoscopic lobectomy. Thorac Surg Clin 2008;18:259-62. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9; discussion 50. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Hanna JM, Berry MF, D'Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis 2013;5:S182-9. [PubMed]
- Stoelben E, Ludwig C. Chest wall resection for lung cancer: indications and techniques. Eur J Cardiothorac Surg 2009;35:450-6. [Crossref] [PubMed]
- Shah AA, D'Amico TA. Primary chest wall tumors. J Am Coll Surg 2010;210:360-6. [Crossref] [PubMed]
- Erhunmwunsee L, D'Amico TA. Surgical management of pulmonary metastases. Ann Thorac Surg 2009;88:2052-60. [Crossref] [PubMed]
- Matsuoka H, Nishio W, Okada M, et al. Resection of chest wall invasion in patients with non-small cell lung cancer. Eur J Cardiothorac Surg 2004;26:1200-4. [Crossref] [PubMed]
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-99S.
- National comprehensive cancer. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. 2017.
- Hennon MW, Dexter EU, Huang M, et al. Does Thoracoscopic Surgery Decrease the Morbidity of Combined Lung and Chest Wall Resection? Ann Thorac Surg 2015;99:1929-34; discussion 34-5.
- Demmy TL, Yendamuri S, Hennon MW, et al. Thoracoscopic maneuvers for chest wall resection and reconstruction. J Thorac Cardiovasc Surg 2012;144:S52-7. [Crossref] [PubMed]
- Demmy TL, Nwogu CE, Yendamuri S. Thoracoscopic chest wall resection: what is its role? Ann Thorac Surg 2010;89:S2142-5. [Crossref] [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. Feasibility of hybrid thoracoscopic lobectomy and en-bloc chest wall resection. Eur J Cardiothorac Surg 2012;41:888-92. [Crossref] [PubMed]
- Rosso L, Palleschi A, Mendogni P, et al. Video-assisted pulmonary lobectomy combined with transmanubrial approach for anterior Pancoast tumor resection: case report. J Cardiothorac Surg 2016;11:65. [Crossref] [PubMed]
- Yokoyama Y, Chen F, Aoyama A, et al. Combined operative technique with anterior surgical approach and video-assisted thoracoscopic surgical lobectomy for anterior superior sulcus tumours. Interact Cardiovasc Thorac Surg 2014;19:864-6. [Crossref] [PubMed]
- Shikuma K, Miyahara R, Osako T. Transmanubrial approach combined with video-assisted approach for superior sulcus tumors. Ann Thorac Surg 2012;94:e29-30. [Crossref] [PubMed]
- Nakajima T, Watanabe A, Nakazawa J, et al. Transmanubrial approach with video-assisted thoracoscopic surgery for left superior sulcus tumour with dense adhesion after replacement of descending thoracic aorta. Interact Cardiovasc Thorac Surg 2012;14:906-8. [Crossref] [PubMed]
- Rosso L, Nosotti M, Palleschi A, et al. VATS lobectomy combined with limited Shaw-Paulson thoracotomy for posterolateral Pancoast tumor. Tumori 2016;102. [PubMed]
- Hennon MW, Demmy TL. Thoracoscopic resection and re-resection of an anterior chest wall chondrosarcoma. Innovations (Phila) 2012;7:445-7. [PubMed]
- Gera PK, La Hei E, Cummins G, et al. Thoracoscopy in Chest Wall Ewing's Sarcoma. J Laparoendosc Adv Surg Tech A 2006;16:509-12. [Crossref] [PubMed]
- Ninomiya H, Maeda M, Matsuzaki Y, et al. Giant cell tumor of the rib. Jpn J Thorac Cardiovasc Surg 2002;50:224-6. [Crossref] [PubMed]
- Rocco G, Fazioli F, Martucci N, et al. Video-assisted thoracic surgery rib resection and reconstruction with titanium plate. Ann Thorac Surg 2011;92:744-5. [Crossref] [PubMed]
- Kara HV, Balderson SS, Tong BC, et al. Video assisted transaxillary first rib resection in treatment of thoracic outlet syndrome (TOS). Ann Cardiothorac Surg 2016;5:67-9. [PubMed]
- Seder CW, Rocco G. Chest wall reconstruction after extended resection. J Thorac Dis 2016;8:S863-71. [Crossref] [PubMed]
- Kara HV, Balderson SS, D'Amico TA. Challenging cases: thoracoscopic lobectomy with chest wall resection and sleeve lobectomy-Duke experience. J Thorac Dis 2014;6:S637-40. [PubMed]
- Martinez-Ferro M, Fraire C, Saldana L, et al. Complete videoendoscopic harvest and transposition of latissimus dorsi muscle for the treatment of Poland syndrome: a first report. J Laparoendosc Adv Surg Tech A 2007;17:108-13. [Crossref] [PubMed]
- Wei B, Eldaif SM, Cerfolio RJ. Robotic Lung Resection for Non-Small Cell Lung Cancer. Surg Oncol Clin N Am 2016;25:515-31. [Crossref] [PubMed]
Cite this article as: Kara HV, Keenan JE, Balderson SS, D’Amico TA. Video assisted thoracic surgery with chest wall resection. Video-assist Thorac Surg 2018;3:15.


