
Current status of video-assisted thoracoscopic surgery lobectomy in nodal positive non-small cell lung cancer
Introduction
There has been increasing evidence that video-assisted thoracoscopic surgery (VATS) lobectomy for patients with non-small cell lung cancer (NSCLC) is the preferred approach compared to thoracotomy due to fewer complications and faster recovery (1-3). Initially, VATS was limited to early stage NSCLC because of concern about oncologic equivalence. However, over time, it has been shown that VATS lobectomy is safe for early stage disease with sound oncologic outcomes. VATS is now being applied to patients with bigger tumors, patients with nodal positive disease, and those who have received induction therapy (4-8). In this article, four aspects of VATS lobectomy for patients with node positive tumors will be reviewed. These include nodal clearance and rates of pathological upstaging, the learning curve associated with VATS lobectomy, the use of robotics, and the role of VATS in patients who have received neoadjuvant treatment.
Lymph node clearance and pathological upstaging
Adequate lymph node clearance is an essential part of the surgical management of NSCLC. It helps in assessment of prognosis and determines therapeutic options. Two important measures when comparing VATS versus thoracotomy are the number of lymph nodes harvested and the rate of nodal upstaging. Pathologic nodal upstaging can be used as a proxy for completeness of nodal evaluation. Early studies examining the approaches have shown that thoracotomy retrieved more lymph nodes and had a higher rate of nodal upstaging as compared to VATS (Table 1). Lee and colleagues reported that compared to VATS, thoracotomy yielded more nodes (14.3 vs. 11.3) and removed more nodal stations (3.8 vs. 3.1) (9). Interestingly, they did not find a difference in 3- and 5-year overall survival (OS) or disease-free survival (DFS) between the groups, (87.4% and 76.5% for VATS OS, and 81.6% and 77.5% for thoracotomy OS, respectively). More than 90% of their patients were clinical stage I (Table 2). Denlinger and colleagues analyzed lymph node evaluation in VATS lobectomy compared to thoracotomy in over 500 patients at their institution and found that VATS sampled significantly fewer lymph nodes, 7.4 vs. 8.9, as well as significantly fewer N2 nodes, 2.5 vs. 3.7 (10). In their short follow-up period, the 3-year survival showed no difference (74.5% open vs. 83.3% VATS). Flores and colleagues also reported that VATS removed significantly fewer nodal stations, 3.6 vs. 4.5, when compared to thoracotomy (2). The 5-year survival of the VATS group was 79% which was similar to the thoracotomy group at 75%.
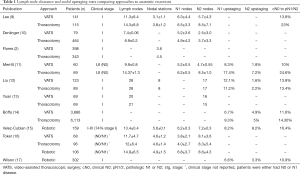
Full table

Full table
Boffa and colleagues queried The Society of Thoracic Surgeons-General Thoracic Database and found 11,500 patients who underwent anatomic resection for clinical stage I NSCLC (14). They reported that the VATS lobectomy group had a lower rate of N1 upstaging compared with the thoracotomy group (6.7% vs. 9.3%, respectively), suggesting that VATS may compromise hilar and peribronchial nodal clearance. Of note, the N1 upstaging rates were similar when comparing thoracotomy patients to patients undergoing minimally-invasive lobectomy by experienced VATS surgeons. Merritt and colleagues reviewed their institutional data comparing thoracotomy to VATS for N0 NSCLC and found that in their thoracotomy group, significantly more lymph nodes were dissected, 14.6 vs. 9.9 nodes (11). They also found a higher rate of nodal upstaging to N1/N2, 24.6% vs. 10%. The 3-year survival between both groups showed no difference (89.9% VATS vs. 84.7% open). In the end, while the data in these studies show that VATS may harvest fewer lymph nodes and sample fewer nodal stations, it does not necessarily translate into worse survival.
There have been other studies suggesting that VATS is comparable to thoracotomy when it comes to lymph node clearance. Liu and colleagues reviewed 212 consecutive lobectomies at their institution comparing VATS to thoracotomy (12). They reported an equal number of lymph nodes removed (28 nodes), an equal number of lymph node stations dissected (8 stations) and an equal number of N2 nodes resected (17 nodes). Their rate of nodal upstaging was 13.8% for VATS and 13.4% for thoracotomy lobectomy. They found no differences in both their 3- and 5-year OS and DFS between the approaches. Yuan and colleagues analyzed 138 patients with clinical stage I disease, divided into propensity-matched groups, who underwent lobectomy by either VATS or thoracotomy (13). The number of dissected lymph nodes was similar (20 nodes for VATS vs. 21 nodes for thoracotomy) as well as the number of N2 nodes harvested (16 nodes for VATS versus 15 nodes for thoracotomy). OS and DFS survival at 5 years, like most studies, were similar.
The VATS learning curve
An important factor in nodal clearance rates and nodal stations removed has been the learning curve associated with adoption of VATS lobectomy. It has been reported that the initial learning curve for VATS lobectomy is anywhere between 50 to 100 cases (18-20). Once an individual surgeon surpasses this level, it has been demonstrated that there is an increase in the number of lymph nodes harvested and the number of lymph node stations removed. Lee and colleagues reviewed their institutional data and found that across 500 consecutive patients, the total number of lymph nodes removed, 11 vs. 12, and lymph node stations removed, 3 vs. 4, increased as they gained cumulative experience (21). They also recognized that, with experience, they performed VATS on older patients, often with more compromised pulmonary function and more advanced stage disease. Despite this, the propensity-matched early and late groups found no difference in DFS at 3 years, 82% in the early group vs. 85% in the late group. Gonzalez-Rivas and colleagues similarly analyzed their initial 3 years of VATS lobectomies in 200 patients (22). They reported a significant improvement in number of nodes harvested, 11.9 nodes during the first year compared to 13.9 in the third year. They also found that more nodal stations were explored, 3.6 stations during the first year compared to 4.5 stations during the third year, with increased experience. Chen and colleagues recently reported a propensity-matched comparison of VATS to thoracotomy lobectomy for patients with clinical stage II and IIIa NSCLC with 120 patients in each arm (23). Evaluating the thoroughness of lymph node clearance, they found that compared to their early group, a greater number of nodes (16.5 vs. 12.2), total nodal stations (5.8 vs. 5.2), N1 stations (2.4 vs. 1.8), N1 nodes (5.7 vs. 4.3) and N2 nodes (10.8 vs. 8.0) were retrieved by VATS than thoracotomy in their late group. With greater cumulative experience and the continued maturation of VATS techniques by thoracic surgeons, VATS can be safely applied to the majority of cases without compromising nodal clearance rates.
Robotic-assisted VATS lobectomy
Over the last decade, the robotics platform has been increasingly applied to thoracic surgery. Some of the benefits over traditional VATS include three-dimensional visualization, fully articulating instrumentation, 10× magnification, computer-assisted scaled motion, and reduction of hand-related tremors (24). These advantages potentially facilitate a more complete nodal harvest due to the more precise dissection of the mediastinal and hilar lymph nodes. This aspect of robotic-assisted VATS (RVATS) lobectomy is worth highlighting since a more complete nodal dissection ultimately allows for an increased ability to identify occult metastatic disease in the locoregional lymph nodes. In patients with clinically positive nodal disease, lymph node clearance becomes even more important to remove all existing and potential disease. Velez-Cubian and colleagues reviewed 159 robotic-assisted lobectomies for NSCLC, examining their efficacy of lymph node dissection (15). They reported their mean total of lymph node stations assessed was 5.6 stations while their mean total lymph nodes dissected were 13.4 lymph nodes. Ninety-eight point one percent of their patients had ≥3 N2 lymph node stations assessed, which the current National Comprehensive Cancer Network guidelines suggest is the minimum N2 stations needed for accurate staging (25). Their rate of nodal upstaging was 19%. They concluded that lymph node dissection during RVATS lobectomy was effective and comparable, if not at times better, than historical data published for traditional VATS and thoracotomy approaches. Toker and colleagues compared their data for open versus VATS versus robotic-assisted approaches in the dissection of N1 and N2 lymph nodes during lung resection (16). They found the robotic approach dissected more lymph nodes compared to VATS and open (14.9 vs. 11.7 vs. 12, respectively), with the increase being in the N1 nodes at levels 11 and 12. The number of N1 and N2 lymph node stations assessed were similar between the approaches (4.6 for VATS, 4.6 for open, 4.9 for RVATS). Wilson and colleagues looked at the prevalence of nodal upstaging during robotic anatomic lung resections for stage I NSCLC in 302 patients (17). They were able to demonstrate a 6.6% rate of N1 upstaging and a 3.3% rate of N2 upstaging for a total of 10.9% rate of nodal upstaging. This was comparable to published literature for VATS but was less than rate of upstaging with thoracotomy. As the robotic technology continues to develop and RVATS lobectomy techniques continue to be refined, it can likely be applied to patients with nodal positive NSCLC given the potentially superior nodal clearance rate.
Role of VATS after induction therapy
Induction therapy plays an important role in patients with locally advanced NSCLC with nodal positive disease. Patients who are found to have mediastinal nodal involvement during pre-resection staging are typically given induction chemotherapy with or without radiation. In some cases, induction therapy is given to patients with bulky hilar nodal disease. There is concern that induction therapy causes an inflammatory response to the lung and hilar structures, leading to adhesions and difficulty clearing the mediastinal lymph nodes. While this was previously considered to be a relative contraindication to a VATS approach, VATS has been demonstrated to be safe and effective for patients receiving neoadjuvant treatment for NSCLC (4,26,27). Patients who receive induction therapy and proceed to VATS have a higher rate of conversion to open which may increase the length of stay but typically does not increase the rate of postoperative complications. Huang and colleagues evaluated 43 patients with stage IIA-IIIB NSCLC who underwent induction therapy (chemotherapy, targeted therapy and radiotherapy, either alone or in combination) followed by VATS resection including 28 lobectomies (4). They reported a 16.7% conversion rate, a 9.5% complication rate with 1 and 3-year survival rates of 94% and 65%, respectively (Table 3). They harvested a mean of 16.88 lymph nodes. They concluded that VATS following neoadjuvant therapy is safe and feasible for the treatment of locally advanced NSCLC and that the long-term efficacy was satisfactory. Kamel and colleagues reviewed their experience with patients that underwent lobectomy after induction therapy, comparing VATS to thoracotomy (28). A 1:2 propensity match was performed with 40 VATS patients and 74 thoracotomy patients. Induction therapy given was either conventional chemotherapy, one of three targeted therapies in a clinical trial, or conventional chemotherapy combined with a COX-2 inhibitor. No differences were found in the number of lymph nodes resected (12 vs. 15, P=0.94), the number of stations (4 for each group), or in the rate of R0 resections (95% vs. 96%) comparing VATS to thoracotomy groups. The rate of open conversion was 12.5%, which was secondary to dense adhesions. 5-year DFS showed no difference between the VATS and thoracotomy groups (73% vs. 48%, P=0.09). They also found that the VATS approach was associated with less estimated blood loss, shorter length of stay and a trend towards fewer postoperative complications.

Full table
Gonzalez-Rivas and colleagues looked at 43 patients who underwent uniportal VATS resections for locally advanced NSCLC, including 37 lobectomies, of whom approximately two thirds had neoadjuvant treatment (chemotherapy vs. chemoradiotherapy) (7). Their reported a conversion rate was 6.5%, a complication rate of 14% and a 30-month survival rate of 74%. They harvested, on average, 16.5 lymph nodes and assessed 4.97 nodal stations. They concluded that uniportal VATS in locally advanced NSCLC, including those who received neoadjuvant treatment was safe and reliable. Yang and colleagues reviewed 272 patients looking at long-term survival following open vs. VATS lobectomy after neoadjuvant chemotherapy ± radiation for NSCLC (27). They found that VATS trended toward improved survival on multivariate analysis but did not reach statistical significance. Their open conversion rate was 10%, five patients due to adhesions while two were due to bleeding. On their propensity-matched analysis, they reported that the VATS approach compared to thoracotomy had a similar 3-year OS (54% vs. 49%) and DFS (34% vs. 24%). They concluded that overall, VATS was safe for patients who received induction therapy without compromising oncologic outcomes.
Conclusions
VATS lobectomy has been increasingly shown to be safe, feasible and oncologically sound for both early and advanced stage NSCLC. Whether the patient has a large tumor, nodal positive disease or received neoadjuvant treatment, VATS lobectomy may be offered to the patient if it is technically appropriate. As our cumulative experience with both VATS and robotic-assisted approaches increases, so does our ability to provide an adequate and thorough mediastinal and hilar lymph node dissection. It is essential that we as thoracic surgeons continue to push ourselves and embrace minimally invasive techniques, with the goal of offering to our lung cancer patients the best oncologic resection with the least amount of associated complications and recovery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Todd Demmy) for the series “VATS for Locally Advanced Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2018.03.06). The series “VATS for Locally Advanced Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McKenna RJ, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 5-6. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 1999;68:194-200. [Crossref] [PubMed]
- Huang J, Xu X, Chen H, et al. Feasibility of complete video-assisted thoracoscopic surgery following neoadjuvant therapy for locally advanced non-small cell lung cancer. J Thorac Dis 2013;5:S267-73. [PubMed]
- Nakanishi R, Fujino Y, Yamashita T, et al. Thoracoscopic anatomic pulmonary resection for locally advanced non-small cell lung cancer. Ann Thorac Surg 2014;97:980-5. [Crossref] [PubMed]
- Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [PubMed]
- Nakano T, Endo S, Endo T, et al. Surgical Outcome of Video-Assisted Thoracoscopic Surgery vs. Thoracotomy for Primary Lung Cancer >5 cm in Diameter. Ann Thorac Cardiovasc Surg 2015;21:428-34. [Crossref] [PubMed]
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951-60; discussion 60-1. [Crossref] [PubMed]
- Denlinger CE, Fernandez F, Meyers BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg 2010;89:1730-5; discussion 6.
- Merritt RE, Hoang CD, Shrager JB. Lymph node evaluation achieved by open lobectomy compared with thoracoscopic lobectomy for N0 lung cancer. Ann Thorac Surg 2013;96:1171-7. [Crossref] [PubMed]
- Liu C, Li Z, Bai C, et al. Video-assisted thoracoscopic surgery and thoracotomy during lobectomy for clinical stage I non-small-cell lung cancer have equivalent oncological outcomes: A single-center experience of 212 consecutive resections. Oncol Lett 2015;9:1364-72. [Crossref] [PubMed]
- Yuan J, Dai G, Kong F. Long-term outcomes of video-assisted thoracoscopic versus open lobectomy for non-small-cell lung cancer with propensity score matching. Int J Clin Exp Med 2016;9:3572-8.
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 53. [Crossref] [PubMed]
- Velez-Cubian FO, Rodriguez KL, Thau MR, et al. Efficacy of lymph node dissection during robotic-assisted lobectomy for non-small cell lung cancer: retrospective review of 159 consecutive cases. J Thorac Dis 2016;8:2454-63. [Crossref] [PubMed]
- Toker A, Özyurtkan MO, Demirhan Ö, et al. Lymph Node Dissection in Surgery for Lung Cancer: Comparison of Open vs. Video-Assisted vs. Robotic-Assisted Approaches. Ann Thorac Cardiovasc Surg 2016;22:284-90. [Crossref] [PubMed]
- Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901-6; discussion 6-7.
- Petersen RH, Hansen HJ. Learning curve associated with VATS lobectomy. Ann Cardiothorac Surg 2012;1:47-50. [PubMed]
- McKenna RJ. Complications and learning curves for video-assisted thoracic surgery lobectomy. Thorac Surg Clin 2008;18:275-80. [Crossref] [PubMed]
- Carrott PW, Jones DR. Teaching video-assisted thoracic surgery (VATS) lobectomy. J Thorac Dis 2013;5:S207-11. [PubMed]
- Lee PC, Kamel M, Nasar A, et al. Lobectomy for Non-Small Cell Lung Cancer by Video-Assisted Thoracic Surgery: Effects of Cumulative Institutional Experience on Adequacy of Lymphadenectomy. Ann Thorac Surg 2016;101:1116-22. [Crossref] [PubMed]
- Gonzalez D, de la Torre M, Paradela M, et al. Video-assisted thoracic surgery lobectomy: 3-year initial experience with 200 cases. Eur J Cardiothorac Surg 2011;40:e21-8. [Crossref] [PubMed]
- Chen K, Wang X, Yang F, et al. Propensity-matched comparison of video-assisted thoracoscopic with thoracotomy lobectomy for locally advanced non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;153:967-76.e2. [Crossref] [PubMed]
- Wei B, D'Amico TA. Thoracoscopic versus robotic approaches: advantages and disadvantages. Thorac Surg Clin 2014;24:177-88. vi. [Crossref] [PubMed]
- Network NCC. Clinical Practice Guidelines - Non-Small Cell Lung Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Petersen RP, Pham D, Toloza EM, et al. Thoracoscopic lobectomy: a safe and effective strategy for patients receiving induction therapy for non-small cell lung cancer. Ann Thorac Surg 2006;82:214-8; discussion 9. [Crossref] [PubMed]
- Yang CF, Meyerhoff RR, Mayne NR, et al. Long-term survival following open versus thoracoscopic lobectomy after preoperative chemotherapy for non-small cell lung cancer. Eur J Cardiothorac Surg 2016;49:1615-23. [Crossref] [PubMed]
- Kamel MK, Nasar A, Stiles BM, et al. Video-Assisted Thoracoscopic Lobectomy Is the Preferred Approach Following Induction Chemotherapy. J Laparoendosc Adv Surg Tech A 2017;27:495-500. [Crossref] [PubMed]
Cite this article as: Le NM, Lee PC. Current status of video-assisted thoracoscopic surgery lobectomy in nodal positive non-small cell lung cancer. Video-assist Thorac Surg 2018;3:13.


