Value of nonintubated thoracoscopic biopsy for mediastinal masses
Introduction
Masses of the mediastinum may arise from a wide range of pathologic conditions (1). They require prompt and correct diagnosis through the collection of large samples of tissue in order to establish appropriate and immediate therapy (2). The tissue retrievable by computed tomography (CT)-guided fine needle biopsy may be often inadequate or insufficient for a correct histological diagnosis (3). On the contrary, given its limited invasiveness and optimal diagnostic-yield, video-assisted thoracoscopic surgery (VATS) has progressively gained a more important role in these conditions (4). However, the execution of VATS implies traditionally the employ of general anesthesia and one-lung ventilation, which can induce several adverse effects in these patients, eventually leading to prolonged postoperative ventilation support and a morbidity rate as high as 20% (5-9).
In order to resolve this important hinder more than 15 years ago we started a program of VATS in awake patients (10,11). This—to our knowledge—is the oldest surgical program specifically created for this purpose. Initially, we performed these procedures using a thoracic epidural anesthesia and carrying out the biopsy through three ports (12,13). With the better knowledge of physiopathology, the introduction of novel drugs and the increased skill of operating despite a “breathing” lung, we have successively shifted to a unique access using only local intercostal bloc (14).
The combination uniportal VATS and nonintubated anesthesia couples the advantages of minimal surgical invasiveness with the lesser anesthesiological impact thus achieving better results in both short and long terms compared to classic techniques. In this study we described technical aspects and investigated the feasibility, safety, and efficacy of nonintubated uniportal VATS mediastinal biopsy comparing it with the traditional multiportal VATS one.
Methods
Since October 2001 to August 2017 a total of 213 consecutive patients underwent nonintubated biopsies of mediastinal masses that embodies the only official series of the Awake Thoracic Surgery Research Group from Tor Vergata University. Until July 2006, 68 cases were approached through a multiport access; starting from that date onwards in all the last 145 individuals we used a uniportal access. Before the operation all patients had released their fully informed consent to nonintubated surgery and the possibility of switching to general anesthesia with one-lung ventilation during the procedure.
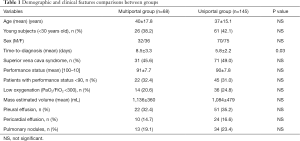
After Institutional Review Board approval and informed consent released by the patients we have retrospectively analyzed the study population. Demographic data are summarized in Table 1.
Full table
Patient selection
In all instances the decision of operating under a nonintubated modality was established after a panel discussion including surgeons, anesthesiologists, intensivists, hematologists, pathologists, radiologists and psychologists. All patients who presented an undetermined mass in the mediastinum requiring surgical biopsy, in the absence of more superficial reachable sites, were considered for nonintubated VATS approach.
Exclusion criteria were imaging-based suspicion of dense pleural adhesions, especially when associated to a clinical history of previous pleural-pulmonary infections or previous thoracic surgery procedures. Another important contraindication to VATS biopsy was the presence of incorrigible coagulation disorders that could favor prolonged bleeding. Ultimately, we also tested the neuro-psychiatric profile of the subject scheduled for nonintubated biopsy in order to exclude the presence of severe anxiety or other conditions (i.e., epilepsy) potentially altering patient’s full cooperation.
Preoperative workup
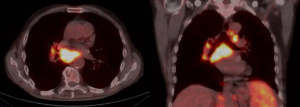
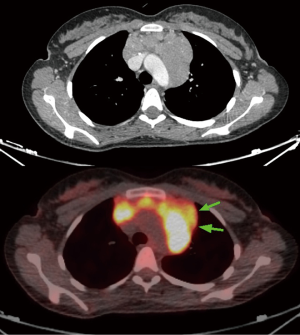
First of all, an accurate evaluation of all superficial lymph nodes suitable to surgical biopsy is advisable before proceeding to an intrathoracic procedure. In the initial part of our experience a CT-guided biopsy, whenever feasible, had been routinely performed and thus surgical option was only considered in the case of non-diagnostic results. However, with the confidence acquired in nonintubated surgery, CT-guided biopsies were restricted to the most compromised patients only. Echocardiography is always used in these patients in order to assess the contractile function of the heart and the presence of effusions. Performance status of the patient scored with the Karnofsky index (100 best status–10 worst status) (15) is all the time assessed before surgery. Radiologic assessment always includes thoracic CT that allowed with the help of a specific algorithm to estimate the volume of the neoplastic tissue. Since 2005 we always associate a positron emission tomography (PET) in order to evidence the most metabolic-active areas and better finalize the biopsies (Figure 1). This implies that all patients in the uniportal group but only a few from the multiportal one underwent this diagnostic procedure. Preoperative administration of steroids is always avoided given the possibility of artifacts in the biopsy samples. Fluids are administered with caution and sometimes low-dose diuretics are also employed to prevent pulmonary and cerebral edema.
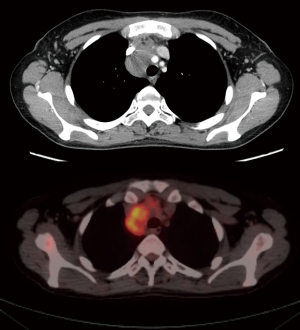
The decision of which side to approach is based on the position of the most suitable area to biopsy of the mass, the safe distance from vascular structures, the location of the most PET-positive tissue, the radiological or anamnesis’ suspect of pleuro-pulmonary adhesions, the presence of pleural effusion or pleural deposits or lung parenchyma lesions (Figure 2).
Setting
As usual for nonintubated thoracic surgery all patients are adequately monitorized with electrocardiogram, pulse oxymeter, systemic and central venous blood pressure, body temperature, arterial line, and end-tidal CO2 by insertion of one detector into a nostril. The surgeon and the anesthesiologist usually establish with the patient a verbal feedback in order to monitor the level of anesthesia and to update about the progress of the procedure. Perception of a calm and professional environment may have a very high impact on patient mood. All patients are warned about the discomfort due to the open pneumothorax, which is generally well tolerated. Anyway, a chest tube with a water seal system is always kept ready on the instrument table.
Anesthesiologist should stay close to the patient’s airways keeping ready the equipment for rapid double lumen intubation. Timed control of blood gases at the various phases of the operation is needed. Furthermore, objective intraoperative monitoring of the effect of sedative drugs on neurological function may be also carried out with the bispectral index (16).
The patient is always lying in lateral decubitus position with mild trunk elevation and both arms kept moderately raised and frontally abducted. The operator usually stands behind or in front of the patient according to the location, anterior or posterior, of the mass in the mediastinum, respectively. The assistant stays aside the surgeon whereas the scrub nurse is located on the opposite side. As usual, monitor and video devices are always placed at the head of the patient but slightly backward in order to leave adequate space to anesthesiologist activity.
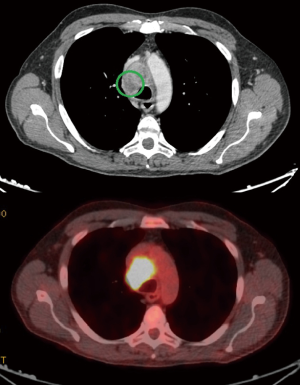
We always use a 10 mm, 30° angled high-definition video-thoracoscope. This kind of surgery does not require very elaborate instruments and the basic curved ring-forceps are adequate for lung retraction. Similarly, electrified hook is an optimal tool to open mediastinal pleura and traditional diathermocautery is quite enough for the progress of the operation. Nevertheless, energy delivering systems such as harmonic (Ultracision®) or radiofrequency (Ligasure®) scalpels may be useful especially in dividing incidental pleuro-pulmonary adhesions and controlling moderate parietal and mediastinal hemorrhages. Biopsy is performed by dedicated forceps with a long-tiny stem and a robust 5 mm spoon-shaped cutting bite able to penetrate into the mass and to remove enough tissue with double action of traction and torsion. Masses are not usually much vascularized and post-biopsy hemorrhage is usually controlled with the simple pressure and without need of instrumental coagulation, which might interfere with further biopsies. In the case of persistent blood spillage we find useful the use of artificial patches of collagen (Hemopatch®). We routinely perform at least three biopsies, 1–9 mm3 in volume, from different sites of the mass chosen according to the visual appearance, palpation consistence and PET positivity (Figure 3). The specimens are always sent for frozen section in order to have immediately information about the appropriateness of the biopsy. Due to the nonintubated anesthesia, the accomplishment of the frozen section does not require supplementary administration of drugs. The operative time is not prolonged unless in the case of a negative response. A second biopsy is performed in these instances.
Multiportal technique
In multiportal technique the anesthesia was administrated through a thoracic epidural catheter inserted at T4 after premedication of 7.5 mg midazolam. Furthermore, patients received a continuous infusion of ropivacaine 0.5% and sufentanil 1.66 µg/mL into the epidural space. In some instances, a topical vagal blockade was also performed.
The operation was carried out through three flexible thoracoscopic trocars, one for the operative thoracoscope usually placed at the sixth intercostal space along the midaxillary line for the camera and the others sited at the fifth intercostal space along anterior axillary line and at the fourth intercostal space along the posterior axillary line. These two last incisions were used alternatively for retracting the lung and for accomplishing the biopsy according to the position of the mass within the mediastinum.
At the end of the procedure, 28 CH chest tube was inserted through the lowest incision. Lung re-expansion was achieved under thoracoscopic vision by asking the patient to breathe deeply and cough repeatedly. The epidural catheter was usually removed on postoperative day one.
Uniportal technique
In uniportal technique all patients undergo a mixed anesthesia including local intercostal bloc, intravenous infusion of opioids and aerosolized 5 mL solution of 2% lidocaine to avoid cough reflex. The intercostal bloc is accomplished by injection of 10 mL 7.5% ropivacaine around the nerve of the selected space after separate local infiltration of 10 mL 2% lidocaine, for achieving both rapid onset and long duration of the analgesic effect. Site of infiltration is done along the space selected for uniportal VATS and included subcutaneous layers, intercostal nerve and parietal pleura. In addition benzodiazepine (midazolam 0.03–0.1 mg/kg) or opioids (remifentanyl 15 µg/kg/min) are intravenously supplemented during lung manipulation or biopsy.
The unique incision is usually made at the fifth intercostal space along the anterior axillary line, 3–4 cm long, under the submammary line in order to be easily hidden underneath the bra in the female subjects. This incision allows reaching many mediastinal regions with the introduction of many instruments and it is protected by a self-standing annular retractor (Alexis®, Applied Medical, USA). Camera is usually positioned at the posterior end of the incision, whereas the operative instrument is introduced in the anterior part of the incision. Lung is retracted with a pad or ring forceps. At the end of the procedure chest drainage is positioned at the posterior extremity of the incision and the wound closed following the same method already described for the multiportal VATS.
Postoperative care
After a short stay in the recovery room the patient is send by the anesthesiologist to the ward, where is allowed to drink and eat. Pain according to visual analogue scale (VAS) (0 no pain–10 maximal pain, indicated by the patient) (17) and blood gas analysis were timely assessed. Young or uncomplicated patients are evaluated for same-day discharge according to surgical and anesthesiological criteria to be satisfied. They mainly include stable and satisfactory clinical conditions, good looking 4-hour postoperative chest X-ray, no air leak and less than 100 mL/h of effusion from the drainage, no previous pleural or pericardial effusion and possibility of adequate home assistance. Finally, at one month from the operation the patient was asked to express his satisfaction with the procedure indicating 4 possible scores: 4= excellent, 3= good, 2= satisfactory, 1= unsatisfactory.
Statistics
Statistical analysis was performed using the SPSS package, version 20.0 (IBM, Chicago, Illinois, USA). Descriptive statistics were presented as mean ± standard deviation (SD). Statistical significance was set at 0.05 P values. Comparison between the two groups (multiportal versus uniportal) was prudentially performed with non-parametric tests for dichotomic and continuous variables using Chi square and Mann-Whitney tests, respectively. Accuracy for diagnostic yield was number of true positive and true negative by the total of the observations.
Results
The two groups of patients (multiportal and uniportal) appear homogeneous for both demographic and clinical variables (Table 1). Nearly all subjects were somewhat symptomatic, whereas in 36 instances the mass was an incidental radiological finding. Mean performance status at the Karnofsky index was 91±8 but in 67 instances, it was less than 90. Fifty-one individuals presented a low oxygenation with a PaO2/FiO2 ratio <300.
One hundred and two patients suffered with a classic superior vena cava syndrome with correlated symptoms (i.e., headache and upper limbs-trunk swelling), increment of the venous basal pressure and presence of collateral venous circuits.
In 73 cases there was a prevalently monolateral pleural effusion with 2 chilothorax and 34 presented also a significant pericardial effusion. Forty-seven patients had radiological evidence of pulmonary nodules or opacities. Mean time from clinical suspect and diagnosis was significantly reduced in the uniportal group (P=0.03) (Table 1) and this was due to gained trust among referral hematologists in VATS biopsy. Indeed, 26 had previously received CT-guided biopsy with inconclusive (n=15) or incomplete (n=11) results and these patients were mainly concentrated in the first part of our experience when needle biopsy was always considered before VATS.
Surgical outcome
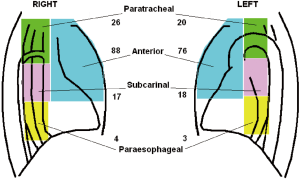
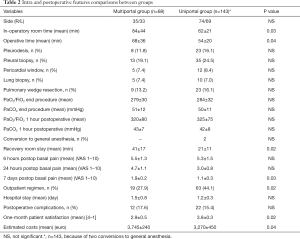
Data regarding surgical outcome are summarized in Table 2. We performed 111 VATS on right and 103 on left side. Biopsies were performed in anterior mediastinum (n=164), subcarinal area (n=35), paratracheal region (n=46) and lower paraesophageal region (n=7), thus in some instances more than one region was taken (Figure 4). Usually, intraoperative oxygenation decreased during the operation reaching a nadir at the end of the procedure but just one hour from the procedure oxygenation reaches the preoperative values. No significant difference was found between groups in PaO2/FiO2 and PaCO2 values (Table 2) at any time.
Full table
We did not experience intraoperative mortality in either group. Both in-operatory room time and operative time were significantly lower in the uniportal group. Conversion to general anesthesia was necessary in two instances; both in uniportal group, and these were due to patient intolerance. We had at least four important episodes of bleeding due to venous mediastinal vessels engorged for vena cava syndrome. In these cases hemorrhage was arrested with the help of clips and Hemopatch without need of conversion.
VATS allowed the contemporary execution of other surgical maneuvers: talc pleurodesis (n=31) mainly in older patients, pleural biopsies (n=48) and, pericardial window (n=17). Furthermore, in the presence of pulmonary lesions 15 lung biopsies and 32 wedge resections were performed.
We experienced postoperative complications in 34 patients (16.0%) and namely cardiac arrhythmias (n=26), pneumonia (n=3), prolonged pleural effusion (n=3) with one case of chilothorax, prolonged air leakage (n=1), epidural hemorrhage (n=1). Complication rate was lower, yet not significantly, in uniportal group (17.6% vs. 15.3%).
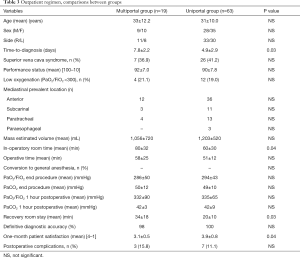
Despite the thoracic epidural anesthesia in multiportal VATS postoperative pain scored by VAS was significantly lower in uniportal group at 24 hours. The difference was even greater 7 days postoperatively. Recovery room stay was significantly shorter in uniportal group. Mean hospital stay was 1.3±0.2 days but very few patients (n=23) required a more than 2 days period. No significant difference in hospital stay between groups was detected. In a total of 82 patients the hospital admission was managed on outpatient basis: multiportal (n=19) and uniportal (n=63) VATS (P=0.02). Data concerning this subset of patients are summarized in Table 3. Finally, both patients’ satisfaction score at one month and estimated costs resulted significantly better in uniportal group (Table 2).
Full table
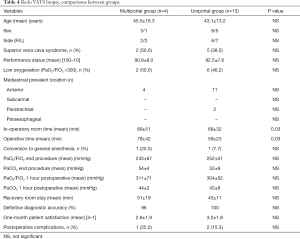
In 17 patients of our series we performed a nonintubated redo VATS through multiportal (n=4) and uniportal (n=13) approach in order to assess partial response after chemotherapy and data are summarized in Table 4. Operation was straightforward in all cases, no significant adhesions were found and adequate biopsy was carried out in all cases. No significant difference was found between groups.
Full table
Diagnostic outcome
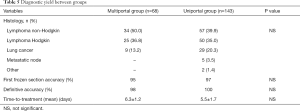
Histology and diagnostic yield are summarized in Table 5. Vast majority was represented by non-Hodgkin (n=91) and Hodgkin’s (n=75) lymphomas, whereas lung cancer were diagnosed in 38 cases. Frozen section examination was positive at the first attempt in 95% of multiportal and 97% in uniportal groups, respectively. Definitive accuracy was 98% in multiportal group and 100% in uniportal group, respectively. This allowed the beginning of the treatment within 7 days in the majority of the patients. No significant differences were evidenced between groups although a shorter time was documented in the uniportal group.
Full table
Discussion
Despite the relative facility of the procedure VATS biopsy of mediastinal mass may become challenging and risky due to the potential and dangerous collateral effects enhanced by one-lung ventilation (5-9). Indeed, a bulky mediastinal masses can cause compression of the airways and the upper venous system and these adverse effects are exaggerated by the supine position. In these conditions general anesthesia can precipitate tracheal patency (6), reduce gas exchanges (8), and decrease pulmonary perfusion (9). Furthermore, one-lung ventilation can create problems related to surgical pneumothorax and trigger a compartmental inflammatory syndrome according to the already described tetralogy: volotrauma, atelectrauma, barotrauma and biotrauma (18).
This is the reason why other procedures such as cervical mediastinoscopy (2) and anterior mediastinotomy or minimalistic biopsies such as those through a CT-guided needle (3) were alternatively preferred to VATS.
Mediastinoscopy and anterior mediastinotomy have been the most commonly used surgical techniques to retrieve mediastinal biopsies (4). They can be usually accomplished in general anesthesia with double lung ventilation although some attempts of performing them under local anesthesia were endeavored (19-22). Actually, these procedures allow only limited visualization with no or poor manageability inside the pleural cavity. Furthermore, they produce quite visible scars whose healing may delay the start of salvage radiotherapy. On the contrary, VATS biopsy can provide better visualization of the targeted mass with adequate bleeding control and multisite mediastinal sampling. At the same time they also permit multi-task intrapleural actions with possibility of performing pleural-pulmonary biopsies and draining concomitant pleural-pericardial effusions (23,24). Lastly, VATS biopsy and especially uniportal VATS creates less visible scars with a better cosmetic outcome compared to both mediastinoscopy and anterior mediastinotomy.
For many years CT-guided fine-needle aspiration or core biopsy given the very low invasiveness has been proposed as initial step when facing a mediastinal mass (3,4). However, the diagnostic yield of this technique sometime does not reach the required accuracy (3,25) particularly low in restaging procedures (26). These unsatisfactory results associated with the possible risks of hypertensive pneumothorax and bleeding and the possibility of missing the diagnosis thus delaying the targeted treatment have progressively reduced the routine employ of this technique.
The development of nonintubated anesthesia and especially the excellent results achieved in other delicate subsets of patients such those affected with emphysema (27) and interstitial lung disease (28) had made the employ of VATS in mediastinal biopsies progressively preferable to the other options. Indeed, in our hands nonintubated VATS mediastinal biopsy was feasible in all patients coupling a negligible morbidity with excellent diagnostic accuracy of nearly 100%.
With the proficiency developed by using the uniportal approach, VATS mediastinal biopsy has now become the first option in the diagnosis of mediastinal masses (11). In the present study we have demonstrated a series of advantages of this approach. First of all, it proved less painful and with a lesser morbidity rate than the multiportal one. The use of intercostal bloc requires less time in the operative room and likely causes less morbidity than thoracic epidural anesthesia and yet providing adequate pain coverage. This approach allowed patient admission regimen based on outpatient basis in almost one half of the cases. The better acceptance from both hematologists and patients permitted to avoid CT-guided biopsy with a reduced the time-to-diagnosis. Furthermore, the faster in-operatory room stay, operative time and recovery room stay make this approach significantly more economic than the multiportal one.
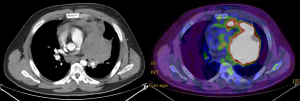
A special discussion deserves the better diagnostic yield. Indeed, all patients from the uniportal group have performed a preoperative PET. In many mass we found areas more active than others and these findings have guided our biopsies (Figures 5-8). This caveat may have influenced the diagnostic yield, which resulted somewhat better, even though not significantly, in both first frozen section and definitive examination. This particular positive outcome might reduce the time to treatment of these patients, which is considered a primary endpoint in this kind of surgery.

Despite the valuable size of the population and the single center origin of the data, the retrospective nature of this investigation distributed on a so wide time span represents a severe limit to interpret this study. It is obvious that many results of the comparison may be affected by the different periods assigned to the two techniques. The most evident example is the use of PET, which was minimal in the multiportal VATS period and might have affected the diagnostic yield as previously mentioned. However, due to the non relevant evolution of postoperative drugs the outcomes concerning the postoperative pain might have some values. An incontrovertible point remains the increased patient acceptance, which is not an insignificant achievement.
We can conclude that the lesser anesthesiological risks of nonintubated VATS biopsy allowed the recruitment of patients previously scheduled to mediastinoscopy and anterior mediastinotomy.
The development of uniportal approach under intercostal bloc together with the routine introduction of PET evaluation has increased the diagnostic accuracy and at the same time shortening the operatory room utilization, reducing chronic postoperative pain and morbidity, allowing the majority of the operation to be carried out in outpatient regimen thus ultimately reducing the economical costs.
Acknowledgments
Professor Vincenzo Ambrogi feels deeply in debt with Professor Tommaso Claudio Mineo, his mentor of Thoracic Surgery at the Tor Vergata University, who allowed him to use his personal series of patients for this publication.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Video-Assisted Thoracic Surgery for the series “Non-intubated Thoracic Surgery”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.10.03). The series “Non-intubated Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. TCM served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). After Institutional Review Board approval and informed consent released by the patients we have retrospectively analyzed the study population.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yim AP. Video-assisted thoracoscopic management of anterior mediastinal masses. Preliminary experience and results. Surg Endosc 1995;9:1184-8. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Nofroni I, et al. Mediastinoscopy in superior vena cava obstruction: analysis of 80 consecutive patients. Ann Thorac Surg 1999;68:223-6. [Crossref] [PubMed]
- Agid R, Sklair-Levy M, Bloom AI, et al. CT-guided biopsy with cutting-edge needle for the diagnosis of malignant lymphoma: experience of 267 biopsies. Clin Radiol 2003;58:143-7. [Crossref] [PubMed]
- Fang WT, Xu MY, Chen G, et al. Minimally invasive approaches for histological diagnosis of anterior mediastinal masses. Chin Med J (Engl) 2007;120:675-9. [PubMed]
- Azizkhan RG, Dudgeon DL, Buck JR, et al. Life-threatening airway obstruction as a complication to the management of mediastinal masses in children. J Pediatr Surg 1985;20:816-22. [Crossref] [PubMed]
- Prakash UB, Abel MD, Hubmayr RD. Mediastinal mass and tracheal obstruction during general anesthesia. Mayo Clin Proc 1988;63:1004-11. [Crossref] [PubMed]
- Goh MH, Liu XY, Goh YS. Anterior mediastinal masses: an anaesthetic challenge. Anaesthesia 1999;54:670-4. [Crossref] [PubMed]
- Cho Y, Suzuki S, Yokoi M, et al. Lateral position prevents respiratory occlusion during surgical procedure under general anesthesia in the patient of huge anterior mediastinal lymphoblastic lymphoma. Jpn J Thorac Cardiovasc Surg 2004;52:476-9. [Crossref] [PubMed]
- Béchard P, Létourneau L, Lacasse Y, et al. Perioperative cardiorespiratory complications in adults with mediastinal mass: incidence and risk factors. Anesthesiology 2004;100:826-34; discussion 5A.
- Mineo TC, Ambrogi V. Efficacy of awake thoracic surgery. J Thorac Cardiovasc Surg 2012;143:249-50; author reply 250-1. [Crossref] [PubMed]
- Mineo TC, Tamburrini A, Perroni G, et al. 1000 cases of tubeless video-assisted thoracic surgery at the Rome Tor Vergata University. Future Oncol 2016;12:13-8. [Crossref] [PubMed]
- Mineo TC. Epidural anesthesia in awake thoracic surgery. Eur J Cardiothorac Surg 2007;32:13-9. [Crossref] [PubMed]
- Tacconi F, Rogliani P, Cristino B, et al. Minimalist video-assisted thoracic surgery biopsy of mediastinal tumors. J Thorac Dis 2016;8:3704-10. [Crossref] [PubMed]
- Mineo TC, Sellitri F, Ambrogi V. Nonintubated video-assisted thoracic surgery from multi to uniport approaches: single centre experience. Eur Med J Respir 2016;4:104-12.
- Karnofsky DA, Abelmann WH, Craver LF, et al. The use of the nitrogen mustards in the palliative treatment of carcinoma – with particular reference to bronchogenic carcinoma. Cancer 1948;1:634-56. [Crossref]
- Kissin I. Depth of anesthesia and bispectral index monitoring. Anesth Analg 2000;90:1114-7. [Crossref] [PubMed]
- Price DD, McGrath PA, Rafii A, et al. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983;17:45-56. [Crossref] [PubMed]
- Mineo TC, Tacconi F. From "awake" to "monitored anesthesia care" thoracic surgery: A 15 year evolution. Thorac Cancer 2014;5:1-13. [Crossref] [PubMed]
- Ward PH. Mediastinoscopy under local anesthesia. A valuable diagnostic technique. Calif Med 1970;112:15-22. [PubMed]
- Arom KV, Franz JL, Grover FL, et al. Subxiphoid anterior mediastinal exploration. Ann Thorac Surg 1977;24:289-90. [Crossref] [PubMed]
- Sibert KS, Biondi JW, Hirsch NP. Spontaneous respiration during thoracotomy in a patient with a mediastinal mass. Anesth Analg 1987;66:904-7. [Crossref] [PubMed]
- Rendina EA, Venuta F, De Giacomo T, et al. Biopsy of anterior mediastinal masses under local anesthesia. Ann Thorac Surg 2002;74:1720-2; discussion 1722-3.
- Migliore M, Giuliano R, Aziz T, et al. Four-step local anesthesia and sedation for thoracoscopic diagnosis and management of pleural diseases. Chest 2002;121:2032-5. [Crossref] [PubMed]
- Migliore M. Efficacy and safety of single-trocar technique for minimally invasive surgery of the chest in the treatment of noncomplex pleural disease. J Thorac Cardiovasc Surg 2003;126:1618-23. [Crossref] [PubMed]
- Petranovic M, Gilman MD, Muniappan A, et al. Diagnostic Yield of CT-Guided Percutaneous Transthoracic Needle Biopsy for Diagnosis of Anterior Mediastinal Masses. AJR Am J Roentgenol 2015;205:774-9. [Crossref] [PubMed]
- Gossot D, Girard P, de Kerviler E, et al. Thoracoscopy or CT-guided biopsy for residual intrathoracic masses after treatment of lymphoma. Chest 2001;120:289-94. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Mineo D, et al. Awake nonresectional lung volume reduction surgery. Ann Surg 2006;243:131-6. [Crossref] [PubMed]
- Ambrogi V, Mineo TC. VATS biopsy for undetermined interstitial lung disease under non-general anesthesia: comparison between uniportal approach under intercostal block vs. three-ports in epidural anesthesia. J Thorac Dis 2014;6:888-95. [PubMed]
- Ambrogi V, Schillaci O, Gallina FT, et al. Non-intubated uniportal mediastinal biopsy. Asvide 2017;4:580. Available online: http://asvidett.amegroups.com/article/view/13569
Cite this article as: Ambrogi V, Schillaci O, Gallina FT, Mineo TC. Value of nonintubated thoracoscopic biopsy for mediastinal masses. Video-assist Thorac Surg 2017;2:77.