Minimally invasive bronchoplastic resections
Introduction
Video-assisted thoracoscopic surgery (VATS) recently was shown in a randomized controlled trial to be superior to open lung resections in terms of postoperative pain and quality of life for early stage lung cancer (1). As the technique evolves, more and more reports emerge on more complex VATS procedures like bronchoplastic resections (2-4). In 2009, we started a VATS lobectomy program. With growing experience we were able to complete extended resections by a minimally invasive approach at our institution. With this study, we aimed to review indications and the key steps of the procedure. Moreover, perioperative data including morbidity and mortality are presented. This study was approved by the local Ethics Committee (UN4424).
Methods
Patient demographics
Between 2009 and 2015, 478 patients were scheduled for a VATS anatomical lung resection. We use a standardized three-incision anterior approach as described by Hansen and Petersen (5). Conversion was performed in 26 patients (5.4%) due to various causes: oncologic reasons occurred in 13 patients, bleeding in nine patients, and technical causes in four. A total of 15 (3.3%) out of 452 VATS cases were bronchoplastic resections (2 bronchial sleeve resections, 11 wedge bronchoplastic, 2 simple bronchoplastic). Median age was 57 (range, 17–75) years; more than half of the patients (8/15) were female. Primary lung cancer was the indication for surgery in all patients. Written informed consent was given by all patients. Demographic data are shown in Table 1.

Full table
Indication
Simple bronchoplasties are a helpful tool when a stapler cannot be placed safely due to the proximity of the tumor to the central airway. Free resection margins were confirmed with frozen section and the orifice of the bronchus was closed using interrupted monofilament stitches (3/0–4/0). A wedge bronchoplasty was performed when during bronchoscopy a simple open transection of the bronchus did not seem suitable or frozen section did show positive margins.
Lymph node infiltration of the bronchus detected during surgery was the reason for the right upper lobe sleeve resection and was necessary to achieve tumor free margins.
Surgical technique
Simple bronchoplasty
The lobar bronchus is transected close to the origin from the central airway. The orifice is closed with single interrupted stitches starting at the ends, then placing one centrally in the orifice and extra stitches as needed.
Wedge bronchoplasty
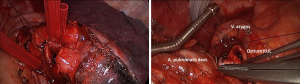
Once, the vein and arterial branches were transected, the bronchial structure was resected in a wedge shape using a scalpel or scissors leaving the posterior bronchial aspect intact. Frozen section was used to check the resection margin. Release maneuvers like dissecting the inferior pulmonary ligament were performed prior to placing the stitches to reduce traction on the anastomosis. Monofilament absorbable sutures (3/0) were used. In most of the cases, an open needle holder was used, as it was easily passed through the minithoracotomy measuring 4 cm. Suturing was started in the corners of the orifice to prevent maladaptation. The next stitch was placed at the middle of the lumen and additional stitches were placed to complete the suture line. In order to avoid tangling of the threads, tourniquets were used for separation (Figure 1). Knot tying was completed using a finger, a round swab or a knot pusher as needed. The suture line was checked for air leaks using a water seal test.
Circumferential bronchoplasty
For the right upper circumferential sleeve resection, the main and intermediate bronchus were transected using a scalpel and scissors. Resection margins were checked with frozen section and the pulmonary ligament was dissected to lower traction on the anastomosis. The anastomosis was completed using interrupted sutures (monofilament absorbable 3/0): the first stitch was placed at the distant edge of the bronchus and the knot was tied immediately. Further stitches were placed in both directions, the cartilaginous part and the membranous part of the bronchus and tied immediately. Once half of the anastomosis was completed, all remaining stitches were placed and tourniquets were used to control the threads. These sutures were tied at the end of the anastomosis. A water seal test was performed to check for air leaks. The anastomosis was covered using the azygos vein (right upper sleeve lobectomy).
Results
Median operative time was 217 minutes (range, 117–390 minutes). All patients were extubated in the operative room. Median chest tube duration was 4 days (range, 2–50 days). Median length of hospital stay was 9 days (range, 6–63 days). There was no in-hospital mortality. Major complications with need for reinterventions occurred in one patient (6.7%) with a chylothorax. The patient had high output over the chest drain and chylomicrons were verified. He refused to undergo re-thoracoscopy after unsuccessful conservative management (oral diet and also npo). Finally, we performed re-thoracoscopy and clipped the thoracic duct. Drainage of chylous fluid stopped and the patient was discharged (pod 63). Minor complication was atelectasis (one patient) with need for bronchoscopy after an upper lobe wedge bronchoplasty to resolve mucus plugging. During the study period, six patients scheduled for a minimally invasive approach had to be converted to open pneumonectomy because of oncologic reasons.
Three patients did undergo induction treatment (chemotherapy only, no radiotherapy) prior to VATS bronchoplastic resection. Final pathology was adenocarcinoma in 4, squamous cell cancer in 5, carcinoid tumors in 4 and other types of histology in 2 patients (mucoepidermoid tumor and large cell tumor). Table 2 summarizes pathologic results and tumor stages. After a median follow up of 26 months, none of the patients had local tumor recurrence. One patient was diagnosed a second lung primary and 3 patients with advanced tumor stages had distant recurrent disease (brain, bone, liver and diffuse pulmonary metastases).
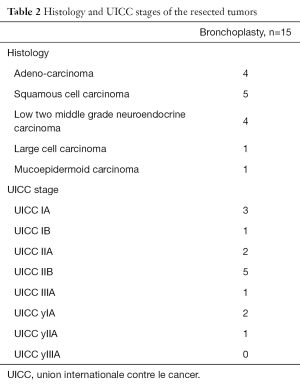
Full table
Discussion
Minimally invasive surgeons have proven on several occasions that a VATS approach for early stage lung cancer is superior to open surgery in many different aspects. Recently, a long awaited prospective randomized trial to show a significant benefit in reducing pain and increasing quality of life was published (1).
Various techniques have been reported with different numbers of incisions; also robotic assisted surgery was established as a valid platform for anatomic lung resections. All of the techniques seem to be reasonable with only little if any difference in postoperative outcome (6,7). With more technical expertise and confidence, the minimally invasive approach is applied to more complex procedures like segmentectomy, pneumonectomy and even bronchoplastic.
In Table 3, various reports on VATS sleeve resections are shown. Concurrent to the definition of VATS lobectomies as totally endoscopic procedures using a number of non-rib-spreading port incisions for safe individual ligation and division of the hilar structures of the lobe with some type of lymphadenectomy (8), several other reports of larger series were excluded in this table.
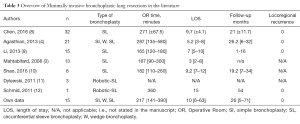
Full table
Different invasion of the tumor towards the central airway require different surgical approaches. We do agree that a simple bronchoplasty is not a real bronchoplastic resection including resection of a central part of the airway. However, bronchial closure with interrupted stitches is technically more challenging than using a stapling device. Moreover, from a training-point of view, it is the first approach to minimally invasive suturing and bronchial anastomosis.
Wedge bronchoplastic have also raised some concern within the literature. A wedge bronchoplasty is technically easier to perform than a circumferential bronchoplasty as it avoids rotation of the remaining lobe. Furthermore, it preserves the blood supply for the airway resulting in better healing and—at least theoretically—in less anastomotic leakage. Recent studies did show oncologic equivalency to sleeve bronchoplastic for small tumors with limited invasion of the bronchus (13).
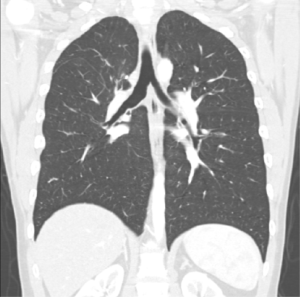
A wedge bronchoplasty usually results in higher tension on the anastomosis than a circumferential sleeve bronchoplasty. It has been suggested, that the largest distance between the upper and lower edge of the bronchus should not be longer than the transverse diameter of the bronchus (13). If this cannot be achieved, a circumferential sleeve should be performed to prevent stenosis of the airway. None of our patients with wedge bronchoplasty did report symptoms suggestive for anastomotic stenosis like coughing, wheezing or recurrent infections. Figure 2 shows a CT scan at the level of the anastomosis, again confirming a wide and non-stenotic airway.
In all our cases, we did use interrupted sutures instead of a running suture. Main reason for this decision was the fact, that tension on the suture is less well controlled in a minimally invasive setting, increasing the risk of anastomotic leakage. However, direct vision via the monitor ensures adequate knot tying and a patent anastomosis. We agree that a running suture might shorten operative time and causes less confusion compared to a high number of loose threads for interrupted sutures. In our setting, the use of tourniquets eased the handling of sutures within the thoracic cavity.
We once again want to stress the fact, that intraoperative frozen section is mandatory for all types of bronchoplastic procedures, as local recurrence can only be treated with pneumonectomy.
Even though arterial sleeve resections are reported in the recent literature, we consider a major tumor invasion of the main pulmonary vessels a contra-indication for a minimally invasive procedure in our department. Performing a proper vascular anastomosis is challenging in open surgery; it needs further refinement of minimally invasive instruments and techniques. For future innovation in minimally invasive thoracic surgery, this might be one of the real advantages of robotic assisted surgery (12,14,15).
Extended VATS procedures need a certain level of training and experience. We performed our first minimally invasive sleeve lobectomy in 2009, completing the anastomosis with the use of a da Vinci robot (12). We did perform the next bronchoplasty after another three years and the experience of almost 200 anatomic VATS resections, indicating the need of minimally invasive skills and confidence to incorporate these extended resections into daily surgical routine. In fairness, we must state, that surgeons in our institution are all well trained in minimally invasive general surgery.
Within the last year, more literature on minimally invasive bronchoplasty was published. Fortunately, a few of these were backed by video material (8). This new way of sharing knowledge will help surgeons all over the world to implement a minimally invasive approach to bronchoplastic procedures.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Luca Bertolaccini and Piergiorgio Solli) for the series “VATS: the age of maturity” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2017.01.03). The series “VATS: the age of maturity” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the local Ethics Committee (UN4424). Written informed consent was given by all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Santambrogio L, Cioffi U, De Simone M, et al. Video-assisted sleeve lobectomy for mucoepidermoid carcinoma of the left lower lobar bronchus: a case report. Chest 2002;121:635-6. [Crossref] [PubMed]
- Mahtabifard A, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32. [Crossref] [PubMed]
- Agasthian T. Initial experience with video-assisted thoracoscopic bronchoplasty. Eur J Cardiothorac Surg 2013;44:616-23. [Crossref] [PubMed]
- Hansen HJ, Petersen RH, Christensen M. Video-assisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc 2011;25:1263-9. [Crossref] [PubMed]
- Perna V, Carvajal AF, Torrecilla JA, et al. Uniportal video-assisted thoracoscopic lobectomy versus other video-assisted thoracoscopic lobectomy techniques: a randomized study. Eur J Cardiothorac Surg 2016;50:411-5. [Crossref] [PubMed]
- Young R, McElnay P, Leslie R, et al. Is uniport thoracoscopic surgery less painful than multiple port approaches? Interact Cardiovasc Thorac Surg 2015;20:409-14. [Crossref] [PubMed]
- Chen H, Huang L, Xu G, et al. Modified bronchial anastomosis in video-assisted thoracoscopic sleeve lobectomy: a report of 32 cases. J Thorac Dis 2016;8:2233-40. [Crossref] [PubMed]
- Li Y, Wang J. Video-assisted thoracoscopic surgery sleeve lobectomy with bronchoplasty: an improved operative technique. Eur J Cardiothorac Surg 2013;44:1108-12. [Crossref] [PubMed]
- Shao F, Liu Z, Pan Y, et al. Bronchoplasty using continuous suture in complete monitor view: a suitable method of thoracoscopic sleeve lobectomy for non-small cell lung cancer. World J Surg Oncol 2016;14:134. [Crossref] [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [Crossref] [PubMed]
- Schmid T, Augustin F, Kainz G, et al. Hybrid video-assisted thoracic surgery-robotic minimally invasive right upper lobe sleeve lobectomy. Ann Thorac Surg 2011;91:1961-5. [Crossref] [PubMed]
- Park SY, Lee HS, Jang HJ, et al. Wedge bronchoplastic lobectomy for non-small cell lung cancer as an alternative to sleeve lobectomy. J Thorac Cardiovasc Surg 2012;143:825-831.e3. [Crossref] [PubMed]
- Augustin F, Bodner J, Maier H, et al. Robotic-assisted minimally invasive vs. thoracoscopic lung lobectomy: comparison of perioperative results in a learning curve setting. Langenbecks Arch Surg 2013;398:895-901. [Crossref] [PubMed]
- Augustin F, Bodner J, Wykypiel H, et al. Initial experience with robotic lung lobectomy: report of two different approaches. Surg Endosc 2011;25:108-13. [Crossref] [PubMed]
Cite this article as: Augustin F, Maier H, Schmid T. Minimally invasive bronchoplastic resections. Video-assist Thorac Surg 2017;2:7.