Influence of mentorship on the duration and safety of robotic learning curve for anatomical lung resections
Introduction
The introduction of the surgical robotic system in 2000 supposed a major advancement in minimally invasive surgery and has become a disruptive technology in surgical practice (1,2). However, the adoption of new technologies such as robotics by practicing surgeons involves an unavoidable learning curve (3). Moreover, there is a belief that learning curves may have a negative impact on patient outcomes which are often worse in earlier phases of the learning curve compared to the later phases (4,5), so that patients may be at a higher risk during the initial period (6), although this hypothesis has been contradicted by the results of a study focus on the implementation period of a robotic program for anatomical lung resection (7). Although some methods have been described to minimize the learning curve, such as training courses, cadaveric resection, and assistance from expert practitioners, ultimately, surgeons must gain proficiency and experience on suitable patients. Therefore, it is critical to monitor the learning process.
On the other hand, despite the fact the first robotic lobectomies were described in 2003 (8,9), the adoption of the robotic technology in thoracic surgery is still limited and its use in lung resections has recently begun to grow. Initial studies have shown that the learning curve for robotic anatomical lung resections can go up to 40–60 procedures (10). These facts highlight the need for new ways to teach new surgical techniques faster decreasing the learning curve without compromising patient safety. In this regard, teaching, coaching and mentorship are widely recognised in training next generation of surgeons (11) and have been proven to be useful in learning new techniques in surgery including robotic procedures (12). Mentorship has been defined as the process whereby an experienced, highly regarded, empathic person (the mentor), guides another individual (the mentee) in the development and re-examination of their own ideas, learning, and personal and professional development. The mentor, who often, but not necessarily, works in the same organisation or field as the mentee, achieves this by listening and talking in confidence to the mentee (13).
Although there is an extensive literature analysing the learning curve for robotic anatomical lung resections (14-20), little work has examined the utility of strategies aimed to minimize its duration and effects on patient care and to date, there has been no study on mentorship association with robotic learning curves or patient safety in thoracic surgery. The primary aim of this study is to evaluate the influence of mentorship on the duration and safety of the learning curve for robotic anatomical lung resection by comparing the learning curves of two different surgeons. We hypothesized that the application of a formal mentorship program decreases the duration of learning curve and improves patient safety during this period.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/vats-21-38).
Methods
Study population
A retrospective cohort study was conducted. We reviewed the prospectively recorded data from 80 anatomical lung resections performed through a robotic approach by two board certified thoracic surgeons with different levels of expertise in two different periods of time; 40 cases corresponded to the first robotic procedures performed by the first surgeon between July 2018 to September 2019 and 40 patients corresponded to the first anatomical lung resections performed by the second surgeon between February 2020 and June 2021.
All patients in both series were 18 years or older, selected for non-extended elective anatomical lung resection (segmentectomy, lobectomy, bilobectomy or pneumonectomy), according to standardized selection criteria (21). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The need for Clinical Research Ethics Committee approval and individual consent was waived according to our institutional law because the study is a retrospective cohort study based on anonymous data of patients.
Surgeons’ expertise and training in robotic-assisted thoracic surgery (RATS)
First surgeon (mentor)
The first surgeon (MFJ) had more than 20 years of surgical practise and a high level of experience in anatomical resections through a posterolateral thoracotomy (>200 procedures) and a muscle-sparing mini-thoracotomy (>200 procedures) and video-assisted thoracic surgery (VATS) (>100 procedures). The first surgeon was Intuitive Surgical certified, and robotic training included da Vinci technology online modules, skill drills with a simulator (>20 h), off-site da Vinci technology training for a console surgeon (two-days course with hands-on sessions on anatomical specimens in the IRCAD centre in Strasbourg, France), and proctorship for the first procedures, but not received mentorship. Before beginning to use the robotic technique for anatomical lung resections, the first surgeon had performed 7 robotic thymectomies. After performing more than 80 robotic procedures, the first surgeon took on the role of mentor for the second surgeon (mentee). During the mentee’s learning period, the mentor continued performing robotic procedures and at the end of June 2021 his current surgical skills included >100 anatomical pulmonary resections with increasing levels of complexity and >30 mediastinal surgeries.
Second surgeon (mentee)
The second surgeon (MTGH) had less than 10 years of surgical practise and an intermediate level of experience in anatomical resections through a posterolateral thoracotomy (>50 procedures), a muscle-sparing mini-thoracotomy (>100 procedures) and VATS (>100 procedures). The second surgeon was Intuitive Surgical certified, and robotic training included da Vinci technology online modules, skill drills with a simulator (>20 h) and off-site da Vinci technology training for a console surgeon (2-days course with hands-on sessions on anatomical specimens in the IRCAD centre). The second surgeon assisted as first assistant surgeon in the surgical field during all robotic procedures performed by the first surgeon, started to use the robotic system after first surgeon mastered the robotic technique and benefited from mentorship of the first surgeon. Before beginning to use the robotic system for anatomical lung resections, the second surgeon had performed 9 robotic thymectomies.
Mentorship activities
Mentorship activities consisted of (I) joint planning of the surgical intervention; (II) direct supervision by the mentor of the procedure performed by the mentee with immediate and constant feedback; (III) guide in the key steps of resection and (IV) joint visualization and analysis of the recording of the surgery.
Operative technique
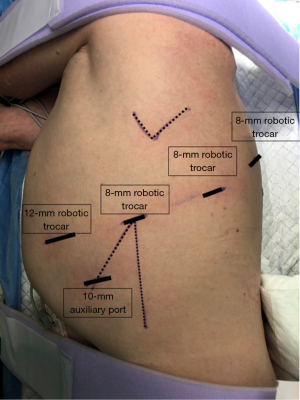
Robotic approach technique was homogenous in all cases: a 4-arm technique was selected. An 8-mm robotic camera trocar was inserted in the eighth intercostal space (ICS) at the mid-axillary line. The cavity was evaluated with the 0° angled camera. A 12-mm robotic trocar was inserted in the eighth ICS at the level of the diaphragm at the anterior axillary line. Two 8-mm robotic trocars were inserted in the eighth ICS at the level of the auscultatory triangle and the scapular line, respectively. Finally, a 10-mm auxiliary port was inserted in the ninth ICS just between the camera port and anterior robotic port establishing a triangle (Figure 1). We used CO2 insufflation pressure of 6 to 10 mmHg. The vessels, the fissure, and the bronchus were divided sequentially, with robotic or manual endo-staplers. Specimen was removed inside of a bag by slightly enlarging the anterior port. At the end, one 24F intercostal drain was placed in through the camera incision.
Perioperative management
Perioperative management was uniform for all patients throughout the study period. Antibiotic prophylaxis consisted in one single dose of cefazoline 2 g, repeated after 6 h if surgery continued. Systematic nodal dissection was performed according to the guidelines of the European Society of Thoracic Surgeons (ESTS) (22). Patients were extubated in the operating room and, after 6 h in the recovery room, transferred to the thoracic ward. At the beginning of the procedure, under direct vision, a paravertebral catheter was inserted for postoperative analgesia with bupivacaine and fentanyl infusion for a maximum of three days postoperatively. Oral paracetamol and non-steroid anti-inflammatory drugs are indicated thereafter. Nursing care was homogeneous in all cases and including incentive spirometry, early mobilisation and standardised intensive physiotherapy is indicated (23).
Statistical analysis
Analysed data included patients’ demographic characteristics. The analysed outcomes were 30-day mortality, operative morbidity, cardiopulmonary complications, operative time, surgical failure and length of hospital stay. Operative morbidity was defined as any postoperative complication occurring during hospitalization or within the first 30 days after the intervention was included: respiratory failure (the need for mechanical ventilation for more than 24 h or the need for reintubation at any time), acute respiratory distress syndrome, atrial arrhythmia, ventricular arrhythmia, atelectasis requiring bronchoscopy, pneumonia, pulmonary thromboembolism, acute myocardial infarction, renal failure, stroke, prolonged air leak, haemothorax, pneumothorax, bronchial fistula, wound dehiscence, wound hematoma, empyema, chylothorax, recurrent nerve paralysis, and phrenic nerve paralysis. Cardio-pulmonary complications were limited to: respiratory failure, need for reintubation, prolonged mechanical ventilation for more than 24 h, pneumonia, atelectasis requiring bronchoscopy, pulmonary oedema, pulmonary embolism, acute respiratory distress syndrome/acute lung injury, arrhythmia requiring treatment, acute myocardial ischemia, acute cardiac failure, stroke/transient ischaemic attacks and acute kidney injury. Surgical failure was defined as any perioperative complication related to technical aspects and included: non-anaesthetic intraoperative complications, conversion to open procedure, reintervention and technical postoperative complications [haemothorax, prolonged air leak (defined as an air leakage into the pleural drainage lasting more than 5 days after surgery), pneumothorax with or without air leak requiring drainage, chylothorax, empyema, recurrent palsy, wound hematoma, wound infection and bronchial fistula]. These complications were already defined according to the joint report of variable definitions agreed by the Society of Thoracic Surgeons and the ESTS (24). Operative time considered time from skin incision to closure including docking and console time. Finally, 30-day mortality was defined as any postoperative death occurring during hospitalization or within the first 30 days after surgery.
Discrete variables were measured as proportions and percentages and compared by Chi-square test or Fisher’s exact test when expected frequencies were below 5. Continuous variables were compared by Wilcoxon’s rank-sum test. Analyses were exploratory in nature and all tests were two-sided, with statistical significance set at a P value of less than 0.05.
Cumulative sum (CUSUM) analysis
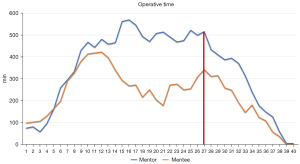
Operative time was analysed by using the CUSUM method determining the running total of differences between the individual data points and the mean of all data points (25). Cases were chronologically arranged from the earliest case to the latest case on the X axis. We calculated for each patient the difference between his individual surgical time and the mean time of all series, calculated the CUSUM of these differences and we represented them graphically on the Y axis. Line 0 in the graph marks the reference value corresponding to the mean of all cases.
Statistical analyses were performed using the statistical software Stata/IC 16.1 (StataCorp., College Station, TX, USA).
Results
Patient characteristics are summarized in Table 1. There were no differences regarding demographic and baseline characteristics of patients in both groups.
Table 1
| Characteristics | Mentor (n=40) | Mentee (n=40) | P value |
|---|---|---|---|
| Age, mean ± SD, years | 62.16±10.86 | 62.3±12.08 | 0.957 |
| BMI, mean ± SD | 26.94±5.47 | 30.17±24.2 | 0.718 |
| ppoFEV1, mean ± SD, % | 78.89±22.04 | 85.9±16.8 | 0.119 |
| ppoDLCO, mean ± SD, % | 70.44±19.06 | 74.87±15.66 | 0.268 |
| Male sex | 20 (50%) | 17 (42.5%) | 0.501* |
| Coronary disease | 0 (0%) | 2 (5%) | 0.494** |
| Arrythmia | 0 (0%) | 1 (2.5%) | 1** |
| CKD | 1 (2.5%) | 1 (2.5%) | 1** |
| Stroke | 0 (0%) | 0 (0%) | 1** |
| Diabetes | 2 (5%) | 5 (12.5%) | 0.432** |
| Hypertension | 9 (22.5%) | 15 (35.0%) | 0.217* |
| Peripheric arteriopathy | 1 (2.5%) | 0 (0%) | 1** |
| Previous malignancy | 18 (45%) | 13 (32.5%) | 0.251* |
| Tumor size >3 cm | 7 (17.5%) | 5 (12.5%) | 0.531* |
| cN1–N2 disease | 2 (5%) | 1 (2.5%) | 1** |
| pN1–N2 disease | 4 (10%) | 3 (7.5%) | 1** |
| Type of malignancy | 0.89* | ||
| Primary neoplasm of the lung | 31 (77.5%) | 31 (77.5%) | |
| Metastases other than lung | 6 (15%) | 5 (12.5%) | |
| No lung cancer | 3 (7.5%) | 4 (10%) | |
| Type of resection | 0.839* | ||
| Lobectomy | 30 (75%) | 29 (72.5%) | |
| Bilobectomy | 1 (2.5%) | 2 (5%) | |
| Segmentectomy | 9 (22.5%) | 9 (22.5%) |
*, P value for Chi-square test; **, P value for Fisher’s exact test. SD, standard deviation; BMI, body mass index; ppoFEV1, predicted postoperative forced expiratory volume in one second; ppoDLCO, predicted postoperative carbon monoxide lung diffusion capacity; CKD, chronic kidney disease.
No pneumonectomies and only “easy” anatomical segmentectomies (a single intersegmental dissection surface) (26) were performed during the study period.
Clinical outcomes and adverse events of both cohorts for this study are summarized in Table 2. No perioperative death was observed in the series. Operative morbidity rate was significantly lower in patients operated by the mentee, while no differences were observed regarding cardiopulmonary complications. Length of hospital stay was significantly shorter in the mentee group. Detailed description of perioperative adverse events considered as surgical failure are described in Table 3. Technical postoperative complications and reintervention rates were higher in the group of patients operated by the mentor.
Table 2
| Outcome | Mentor (n=40) | Mentee (n=40) | P value |
|---|---|---|---|
| Operative time, median [IQR], min | 135 [120–180] | 150 [111.25–180] | 0.73* |
| Surgical failure | 10 (25%) | 4 (10%) | 0.077** |
| Operative morbidity | 11 (27.5%) | 3 (7.5%) | 0.019** |
| Cardiopulmonary complications | 3 (7.5%) | 0 (0%) | 0.241*** |
| Length of hospital stay, median [IQR], days | 3 [3–4] | 2.5 [2–3] | 0.003* |
*, P value for Wilcoxon’s rank-sum test; **, P value for Chi-square test; ***, P value for Fisher’s exact test. IQR, interquartile range.
Table 3
| Adverse event | Mentor (n=40) | Mentee (n=40) | P value |
|---|---|---|---|
| Intraoperative complication, n | 3 (7.5%) | 3 (7.5%) | 1** |
| Bronchial injury | 1 | 0 | |
| Air leak | 1 | 0 | |
| Incorrect vein division | 1 | 0 | |
| Incorrect bronchial division | 0 | 1 | |
| Arterial injury | 0 | 1 | |
| Venous injury | 0 | 1 | |
| Conversion, n | 2 (5%) | 2 (5%) | 1** |
| Bronchial injury | 1 | 0 | |
| Air leak | 1 | 0 | |
| Arterial injury | 0 | 1 | |
| Venous injury | 0 | 1 | |
| Technical postoperative complications, n | 9 (22.5%) | 2 (5%) | 0.023* |
| Haemothorax | 2 | 0 | |
| Pleural effusion | 0 | 1 | |
| Prolonged air leak | 4 | 0 | |
| Chylothorax | 1 | 0 | |
| Empyema | 2 | 0 | |
| Wound hematoma | 2 | 0 | |
| Wound infection | 0 | 1 | |
| Bronchial fistula | 1 | 0 | |
| Reintervention, n | 4 (10%) | 0 (0%) | 0.116** |
| Empyema | 1 | 0 | |
| Haemothorax | 2 | 0 | |
| Bronchial fistula | 1 | 0 |
*, P value for Chi-square test; **, P value for Fisher’s exact test.
The duration of the procedure was longer for the mentee, but not statistically significant (median: 150 vs. 135 min, P=0.73). The prevalence of surgical failure was 25% in the cases intervened by the mentor and 10% in the cases intervened by the mentee (P=0.077). The CUSUM graph for operative time shows estimated a learning curve duration of 27 cases for both surgeons (Figure 2).
Discussion
This study demonstrated that the implementation of a formal mentorship program for robotic anatomical lung resections was associated with increased patient safety during the learning curve. Patients’ outcomes including surgical failure, postoperative complications and length of hospital stay improved significantly in the mentee series despite surgeries were performed by a less experienced surgeon. However, the mentorship program is not associated with a decrease of the duration of the learning curve since CUSUM analyses of the operative time indicates that both surgeons reached the learning curve after performing the same number of cases. These results indicate that subsequent generation of surgeons, but above all, patients, can benefit from surgical mentorship.
In this study, operative time was considered the main variable to determine the duration of the learning curve. Although operative times may vary with the complexity of the individual cases, individual surgical skills and expertise level, chronological plots showed that surgical time decreased along time in the case series of both surgeons. We found no reduction of operative time associated to mentorship, indicating that the adoption of the robotic technique requires individual surgeons going through a complete learning curve even when the surgeon receive the constant feedback and advise from a mentor. Our study demonstrated that around 27 procedures are needed to master the robotic technique and to adapt to the specific features of the robotic system such as endowrist instruments manipulation or absence of haptic feedback. Nevertheless, a multidimensional, complete analysis of learning curves must include also technical competence measured as surgical outcomes and patient safety measured as postoperative adverse events rates. Our results regarding duration of the learning curve agree to those published in a systematic review conducted by Power et al. (10) and operative morbidity rates were similar to those described by Toker et al. (15) (24%) and Meyer et al. (16) (16.8%).
As surgical technology evolves, complex techniques must be mastered, and new surgical skills must be acquired. The implementation of new procedures requires surgeons to integrate new techniques into their practice, and this can have an impact on outcomes (27) and despite the continued need for obtaining new knowledge and learning new skills, the professional and public tolerance for a “learning curve” is much less than in previous decades and a potential excess morbidity associated with the learning curve is no longer acceptable. For that reason, the process of implementing new surgical techniques, must include an evaluation of the learning curve based on objective, measurable outcomes. Additionally, it is thus critical to develop strategies that mitigate the learning curve for safe institutional adoption of the technique. Mentorship could be the key to success in these endeavours. This study aimed to evaluate the influence of mentorship on the robotic learning curve and outcomes of anatomical lung resection by comparing the learning curves of two different surgeons: first surgeon who started the robotic program without mentorship and second surgeon who benefited from the mentorship of the first surgeon once he reached the expertise level in robotic procedures.
Although the importance of mentorship in surgical training is widely recognized (28) and substantial efforts have been made to enhance mentorship opportunities, a recent survey distributed to cardiothoracic surgical trainees showed than more than one third of residents had either no mentor or less effective mentorship (29). We consider that mentorship could ultimately be the best tool for mastering complex professional skills and maturing through a learning curve. However, the success of mentorship is two-sided, with responsibilities for both the mentor and the mentee and this relationship requires time, patience, dedication, and to some degree selflessness (30). In addition to mentorship, the detailed tracking of outcomes is an essential tool for mastering any learning curve.
To our knowledge, this is the first study to look at results of the second generation of adopters of the robotic technique for lung surgery who have undergone a formal mentorship during the learning process. However, the effectiveness of mentorship and formal robotic proficiency skills curriculum in improving the subsequent generations’ learning curve has already been analysed in pancreatic surgery (12,31). Results published by Rice et al. (12) demonstrated that operation time, conversion rates and estimated blood loss decreased across generations without a concomitant rise in adverse patient outcomes suggesting that a mentorship program coupled with a proficiency-based curriculum allows for the safe introduction of less experienced surgeons to robotic pancreaticoduodenectomy without compromising patient safety.
Several limitations need to be considered in this study. Firstly, data come from two surgeons whose expertise levels were different at the moment of initiating the robotic anatomical lung resections. However, although mentee’s overall operative experience level was lower, she assisted all mentor’s procedures during his learning curve, so that when she started her robotic learning curve, she was more familiar with the robotic system functioning. Secondly, the mentor learning curve also reflects the institution’s learning curve since an institutional learning curve goes beyond the mere technical competence of the surgeon to encompass other aspects such as optimal port placement, optimal sequencing, and training of operating room staff. Thirdly, although baseline characteristics of patients were similar in both groups, technical complexity was not assessed. However, as mentor and mentee learning period did not coincide at the same time, we consider bias coming from subjective evaluation of the expected technical complexity of the operation were similar in both surgeons. Moreover, some features strongly associated to technical complexity such as tumoral size and lymph node involvement did not differ among groups.
Conclusions
In conclusion, a formal mentorship allows for the safe introduction of less experienced surgeons to robotic anatomical lung resection by decreasing operative morbidity and surgical failure rates during the learning curve. However, it seems to have no impact on the duration of the learning curve.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/vats-21-38
Data Sharing Statement: Available at https://dx.doi.org/10.21037/vats-21-38
Peer Review File: Available at https://dx.doi.org/10.21037/vats-21-38
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/vats-21-38). MFJ serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from January 2021 to December 2022. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The need for Clinical Research Ethics Committee approval and individual consent was waived according to our institutional law because the study is a retrospective cohort study based on anonymous data of patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morris B. Robotic surgery: applications, limitations, and impact on surgical education. MedGenMed 2005;7:72. [PubMed]
- Shah J, Vyas A, Vyas D. The History of Robotics in Surgical Specialties. Am J Robot Surg 2014;1:12-20. [Crossref] [PubMed]
- Hasan A, Pozzi M, Hamilton JR. New surgical procedures: can we minimise the learning curve? BMJ 2000;320:171-3. [Crossref] [PubMed]
- Hopper AN, Jamison MH, Lewis WG. Learning curves in surgical practice. Postgrad Med J 2007;83:777-9. [Crossref] [PubMed]
- Gofton WT, Papp SR, Gofton T, et al. Understanding and Taking Control of Surgical Learning Curves. Instr Course Lect 2016;65:623-31. [PubMed]
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105-12. [Crossref] [PubMed]
- Gómez-Hernández MT, Fuentes MG, Novoa NM, et al. Similar outcomes after newly implemented rats approach compared to standard vats for anatomical lung resection. A propensity-score matched analysis. Cir Esp (Engl Ed) 2021;S0009-739X(21)00135-4.
- Morgan JA, Ginsburg ME, Sonett JR, et al. Advanced thoracoscopic procedures are facilitated by computer-aided robotic technology. Eur J Cardiothorac Surg 2003;23:883-7; discussion 887. [Crossref] [PubMed]
- Ashton RC Jr, Connery CP, Swistel DG, et al. Robot-assisted lobectomy. J Thorac Cardiovasc Surg 2003;126:292-3. [Crossref] [PubMed]
- Power AD, D'Souza DM, Moffatt-Bruce SD, et al. Defining the learning curve of robotic thoracic surgery: what does it take? Surg Endosc 2019;33:3880-8. [Crossref] [PubMed]
- Lin J, Reddy RM. Teaching, Mentorship, and Coaching in Surgical Education. Thorac Surg Clin 2019;29:311-20. [Crossref] [PubMed]
- Rice MK, Hodges JC, Bellon J, et al. Association of Mentorship and a Formal Robotic Proficiency Skills Curriculum With Subsequent Generations' Learning Curve and Safety for Robotic Pancreaticoduodenectomy. JAMA Surg 2020;155:607-15. [Crossref] [PubMed]
- Oxley J, Standing Committee on Postgraduate Medical and Dental Education. Supporting doctors and dentists at work: an enquiry into mentoring. London: SCOPME, 1998.
- Gómez Hernández MT, Fuentes Gago M, Novoa Valentín N, et al. Robotic anatomical lung resections: Analysis of the learning curve. Cir Esp 2021;99:421-7. (Engl Ed). [PubMed]
- Toker A, Özyurtkan MO, Kaba E, et al. Robotic anatomic lung resections: the initial experience and description of learning in 102 cases. Surg Endosc 2016;30:676-83. [Crossref] [PubMed]
- Meyer M, Gharagozloo F, Tempesta B, et al. The learning curve of robotic lobectomy. Int J Med Robot 2012;8:448-52. [Crossref] [PubMed]
- Song G, Sun X, Miao S, et al. Learning curve for robot-assisted lobectomy of lung cancer. J Thorac Dis 2019;11:2431-7. [Crossref] [PubMed]
- Arnold BN, Thomas DC, Bhatnagar V, et al. Defining the learning curve in robot-assisted thoracoscopic lobectomy. Surgery 2019;165:450-4. [Crossref] [PubMed]
- Baldonado JJAR, Amaral M, Garrett J, et al. Credentialing for robotic lobectomy: what is the learning curve? A retrospective analysis of 272 consecutive cases by a single surgeon. J Robot Surg 2019;13:663-9. [Crossref] [PubMed]
- Zhang Y, Liu S, Han Y, et al. Robotic Anatomical Segmentectomy: An Analysis of the Learning Curve. Ann Thorac Surg 2019;107:1515-22. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Varela G, Ballesteros E, Jiménez MF, et al. Cost-effectiveness analysis of prophylactic respiratory physiotherapy in pulmonary lobectomy. Eur J Cardiothorac Surg 2006;29:216-20. [Crossref] [PubMed]
- Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg 2015;99:368-76. [Crossref] [PubMed]
- Yap CH, Colson ME, Watters DA. Cumulative sum techniques for surgeons: a brief review. ANZ J Surg 2007;77:583-6. [Crossref] [PubMed]
- Oizumi H, Kanauchi N, Kato H, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011;141:678-82. [Crossref] [PubMed]
- Healey P, Samanta J. When does the 'learning curve' of innovative interventions become questionable practice? Eur J Vasc Endovasc Surg 2008;36:253-7. [Crossref] [PubMed]
- Stephens EH, Goldstone AB, Fiedler AG, et al. Appraisal of mentorship in cardiothoracic surgery training. J Thorac Cardiovasc Surg 2018;156:2216-23. [Crossref] [PubMed]
- Reich HJ, Lou X, Brescia AA, et al. Mentorship Effectiveness in Cardiothoracic Surgical Training. Ann Thorac Surg 2021;112:645-51. [Crossref] [PubMed]
- Cohen MS, Jacobs JP, Quintessenza JA, et al. Mentorship, learning curves, and balance. Cardiol Young 2007;17:164-74. [Crossref] [PubMed]
- Al Abbas AI, Wang C, Hamad AB, et al. Mentorship and formal robotic proficiency skills curriculum improve subsequent generations' learning curve for the robotic distal pancreatectomy. HPB (Oxford) 2021;23:1849-55. [Crossref] [PubMed]
Cite this article as: Gómez-Hernández MT, Jiménez MF. Influence of mentorship on the duration and safety of robotic learning curve for anatomical lung resections. Video-assist Thorac Surg 2021;6:33.