
Optimizing the uniportal video-assisted thoracic surgery learning curve in major lung resection
Introduction
Uniportal video-assisted thoracic surgery (VATS) is widely established as a technique to perform a large variety of the thoracic surgeons’ repertoire. While there is no level 1 evidence or long term follow up to show a benefit of this technique over existing minimally invasive approaches, there are small comparative and retrospective studies that suggest it is at least clinically and oncologically equivalent in lung cancer resections (1).
Despite entering guidelines as a recommended approach to lung cancer surgery, uptake of VATS has been relatively slow internationally (2,3). Training the next generation of surgeons in VATS techniques is key in increasing this. The usual steps of a surgeon learning uniportal VATS or multiportal have been recommended by experts as follows (4):
- Visit and observe in a high-volume center;
- Watching videos of the procedure to familiarize the steps;
- Virtual simulators;
- Attending specialist courses hands on courses—animal/cadaver models;
- Proctorship in the local center with regular visits recommended to review learning.
Fifty cases are thought to be the number required to achieve proficiency according to the uniportal lobectomy consensus statement (5) this is supported by McKenna’s proposal in multiport VATS of a minimum 50 VATS lobectomies and 100 minor cases for proficiency (6). The flexion point of the learning curve, however, may be smaller—30 cases for experienced multiport VATS surgeons (7). Indeed, experience in two-port VATS is recommended in surgeons planning to learn uniportal techniques (8).
Volume is also thought to be crucial for improving patient outcomes and training. Definition of volume varies widely between country and article written—but generally a high-volume center is thought to do between 70–150 cases per year and is associated with lower perioperative morbidity and mortality (9,10). A new definition of volume has been sought since the two main thoracic surgery hospitals in Shanghai are now performing over 10,000 major thoracic cases per year (10), even very short periods of observing surgery in these centers is associated with an improvement in VATS proficiency (11). In these ultra-high volume centers the learning curve, for inexperienced uniportal VATS surgeons in a fellowship training programme, is felt to be shorter again with a flexion point around 30 carefully selected cases (12).
Whilst safety and equivalent patient outcomes of multiport VATS lobectomy has been shown in even inexperienced trainees (13), there is no data available to our knowledge with regards to training in uniportal anatomical lung resection.
Here we examine perioperative outcomes in lung cancer resection for a single Thoracic Surgery trainee under the supervision of an experienced uniportal VATS surgeon in a high-volume center.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/vats-19-62).
Methods
We performed a retrospective analysis of prospectively collected data on all uniportal VATS lobectomy and anatomical segmentectomy cases performed by a single trainee. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Following internal review, it was decided to waive the need for ethics approval for the collection, analysis and publication of the retrospective anonymized data for this non-interventional study. All patients were diagnosed and treated according to national guidelines. All participants provided written informed consent to use their anonymized data for research purposes prior to the procedure.
From May 2017 to October 2019 our institution performed around 2,000 thoracic surgery cases with 718 anatomical/major lung resections for lung cancer. Three hundred and sixty-three of these were via a VATS approach. The trainer in this study did 170 cases of anatomical lung resection in this period including those performed by the trainee. The trainer’s experience includes over 700 anatomical lung resections by uniportal VATS including sleeve lobectomy and subxiphoid lobectomy.
Patient selection for surgery was per routine for our institution in keeping with national guidelines (14) and multidisciplinary team meeting discussion. Patients are randomly assigned to surgeon in charge in the outpatient clinic. The choice of surgical approach is decided by the consultant surgeon. Preoperative investigations performed routinely include full blood count, serum biochemistry, computed tomography within 6 weeks prior to surgery, histological diagnostic tests, pulmonary function tests and positron emission tomography (PET)-CT scans. Echocardiogram and advanced assessment such as cardiopulmonary exercise tests were performed in selected cases. We perform radical lymphadenectomy in all cases of anatomical lung resection.
As per Table 1 patient characteristics collected include age, body mass index (BMI), co-morbidities, previous chemoradiotherapy, anticoagulation status and lung function. Operative data collected include operation duration, need for conversion, intraoperative difficulties and cause of consultant needing to scrub. Operation duration was from skin incision to skin closure and included lymph node dissection and meticulous hemostasis. Post-operative data collected include lesion size, number of lymph nodes stations sampled, nodal status, resection margin status, patient length of stay, 30 days survival and post-operative air leak.
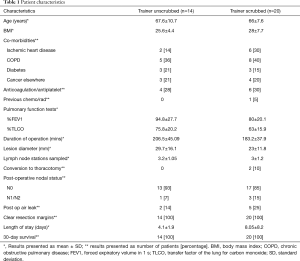
Full table
The study period was the trainee’s initial 7-month training period with a trainer experienced in uniportal VATS. Prior to this, the trainee had completed six cases multiport VATS anatomical lung resection with a consultant unscrubbed, 67 with a consultant assisting, six open Lobectomy cases with a consultant unscrubbed and 15 cases of open lobectomy with a consultant assisting, all in UK centers.
Uniportal lung resection cases were defined as completed by the trainee according to the National Surgical Advisory Committee definition of a case (15). This definition states that the trainee must perform >50% of the dissection of hilum AND divide greater than 50% of the vessels and bronchi.
All cases were performed through a standardized approach, as per the Uniportal VATS Interest Group (UVIG) consensus statement (5), through a single 3–4 cm incision. The main assistants, unless the consultant trainer was scrubbed, were junior trainee surgeons with little experience in either multi or uniportal VATS procedures. The setup/approach is standard for all lobectomy/segmentectomy and has previously been described in detail (16), the technique for segmentectomy dissection is also described (16), while the order of dissection for uniportal lobectomy is similar to that when performed via the subxiphoid approach and has also been described in detail (17).
The consultant trainer early in the learning curve was always present in the theatre by a monitor to teach, advise and scrub when necessary to teach the special movements including retraction of lung, assistant’s positioning, use of energy devices and stapler maneuvers. Later in the learning curve once the trainee felt able to do routine cases unsupervised, the trainer only advised/scrubbed when requested by the trainee but was always available to do so.
All cases were critiqued post operatively to enhance learning. Video record of the cases were edited by the trainee and further comments made by the trainer to cement learning points.
Cumulative sum (CUSUM) analysis
A learning curve is the mathematical representation of the learning process; in our study, we used the operating time to evaluate the trainee’s learning curve. We used CUSUM analysis for the quantitative assessment of the 34 cases. As a sequential analysis method, CUSUM is a particularly good tool to assess learning curves, as it recognizes the importance of time as a ‘hidden variable’. CUSUM is defined as the difference between individual data points and the mean of all data points and therefore is an iterative process. We ordered the cases chronologically from the earliest to the latest and we defined the operation time for each case as OTi and the mean operating time of all the cases as µ. Therefore, the CUSUM at operating time OT is calculated as shown below:
We then plotted the CUSUM against the order of the operations to identify the inflexion point at which the duration of the cases is below the mean operating time for the trainee.
Statistics
The results are presented as mean ± standard deviation for continuous variables and as percentage for categorical variables. To compare the mean durations before and after the inflexion point, we used the Wilcoxon test. R 4.0.0 for Windows was used for statistical analysis and a P value <0.05 was considered statistically significant.
Results
The pre-, peri- and post-operative variable data collected are outlined in Table 1. The types of resection, lobectomy vs. segmentectomy, anatomical location and side, are shown in Table 2. During the training period, of 38 uniportal lung resections four were not completed by the trainee. Two cases with predicted post-operative FEV1 <30% were consultant led. One case completed by consultant due to combination of complexity (upper and lower lobe combined big segmentectomy) in a patient with no fissure and poor lung function. In one early case the trainee dissected extensive adhesions and divided vein but with no fissure present was unable to dissect bronchus/artery.

Full table
Cases allocated to trainer scrubbed are those in which the trainee dissected and divided the majority of vessels/airway and fissure. Table 3 gives the key reason for the consultant decision to scrub into the procedure. Significant intraoperative adhesions were present in seven cases.
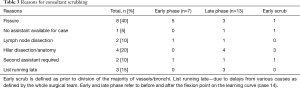
Full table
The trainee completed 14 cases with the consultant trainer unscrubbed for the whole case. The trainer remained available teaching at the screen side in early cases and later monitoring progress or providing reassurance. The UK Surgical advisory definition of a case requires only 50% of hilar dissection and vessel/bronchi division to be completed by the trainee. In the consultant unscrubbed cases the trainee completed 100% of the dissection, division of vessels/bronchi as well as other equally important aspects of the procedure—fissure completion and lymph node dissection.
All patients survived to discharge, there were no conversions for bleeding with the consultant unscrubbed and no significant blood loss in either of the two groups. There were two conversions, one for inability to complete fissure through VATS. The other conversion was for bleeding from the base of the root of the truncus artery on retraction of the lung only, though it was easily controlled and the majority of the case had been completed, it was decided safer to convert in case of the need for more advanced repair.
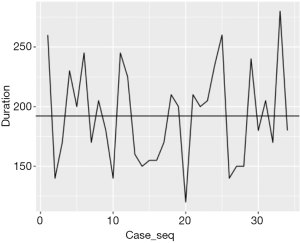
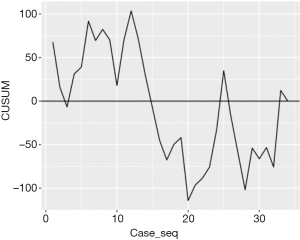
Figure 1 shows the duration of each operation over the case series with the horizontal line demonstrating the mean duration, which is 192 minutes. Figure 2 shows the CUSUM analysis over the course of all the case series. As one would expect, the initial cases were longer than average, whereas the later cases seem to be below the average duration. The inflexion point at which the duration of the operations is below the mean operating time for the trainee happens at case 14. The difference between the mean durations of the first 14 cases and the remaining 20 cases is statistically significant (P value: 3.23×10–8).
Discussion
In this article we have shown that uniportal VATS training can be done safely and with good patient outcomes with a short learning curve. Furthermore, by adapting the learning process, we have shown that a trainee can start to become proficient after performing only 14 cases. The recommended number of cases for surgeons learning VATS lobectomy is 50 in multiport (6) and uniportal (5). Learning a new technique in surgery usually entails either fellowship or mentoring via a proctorship. The former is usually practiced in high volume centers with stepwise case selection as the trainee progresses. Proctorship requires the trainee to observe cases, attend courses including wetlabs before enrolling the services of a proctor to supervise for a small number cases in person before providing mentorship usually at a distance. Several factors influence the length of the learning curve; previous experience in VATS minor or major procedures and learning in higher volume training centers (8,11,18). Here we have shown that with modification to these traditional training methods the learning curve of an inexperienced surgeon can be comparable to that of experienced VATS surgeons (7) and in ultra-high-volume centers (12). The modifications we made are (I) removal of case selection (II) altered role of the trainer (III) use of video editing/review technology to review learning points in the trainees and other surgeons’ operations. This augmented traditional learning the trainer/trainee used such as attendance at wetlab learning days.
Case selection in surgical training may have a reduced role when supervision is more intense. Articles on the learning curve and training in both VATS and non-thoracic specialties support the selection of cases suitable for training (19-21). While our unit is defined as high volume once we divide the cases between the 6 trainer/trainee teams the available cases to the individual trainee begins to dwindle. The minimum resident case volume for a training center in the UVIGs consensus statement is 50 (5). There are, however, no guidelines on how many cases the trainee should complete and over what time frame. Further case selection in our cohort of patients, with a preponderance of lungs difficult to collapse and incomplete fissures, would have made training inefficient due to the lack of cases suitable. Therefore, here, in order to train efficiently we do not select cases. Rather the trainee progresses as far through each case as possible before the trainer provides unscrubbed advice or scrubbed assistance. While removing case selection makes the presence of the consultant trainer in theatre more important to deal with more challenging cases, in lists where easier cases have been selected for trainees, the trainer should always be available to deal with unexpected difficult events. The lack of case selection is reflected in Figure 2, where there are a few cases that took longer than average after the inflexion point (case 14). It was felt that difficult cases offered more enhanced quality of learning and problem solving even if the consultant was required to scrub in to assist or perform parts as happened in 22 cases. This then translated into increased confidence in the 14 cases the trainee performed without the consultant scrubbed, reducing perceived difficulty and presumably shortening the learning curve. With a highly experienced trainer always available this makes training safe and effective. It enabled us to achieve learning curves similar to that of ultra-high-volume centers (12).
We adapted the role of the trainer to be available to provide advice by the screen at any point during the operation and only scrub when screen advice alone was insufficient to progress. The role lies somewhere between that of the Zwisch model of teaching residents and traditional proctorship (22). The Zwisch model requires much more trainer led teaching and operating through stages. Proctorship is limited by the time a proctor can spend with a student. Our method intensified the training experience by making it trainee led but with enhanced supervision. This enabled us to achieve a learning curve similar to an already experienced multiport VATS surgeon (7) but with a reduction in the need for conversion.
In an increasingly stretched national health service, training can be affected by more cases having to be squeezed onto a limited numbers of lists. It was crucial to tailor “training lists” to an appropriate number of cases in agreement with the other theatre staff. To ensure efficiency and support of all theatre staff, during the training programme, checkpoints were instituted to ensure that all members of the team were aware of an operation’s progress. If checkpoints were missed the trainer either intervened with advice or scrubbed to ensure timeliness as happened on three occasions. This also prevents a trainee from becoming frustrated with a lack of progress which can inhibit future learning.
Training should never compromise patient safety and good outcomes are of utmost importance. During the training period there was no major blood loss, perioperative mortality or major complication. Conversion from VATS to open was only done after consultant assessment. Safety has been shown previously in multiport VATS teaching senior trainees (23,24) and even in those with limited open experience (25,26). Having the trainer in theatre by a screen during the initial phase of training allowed good techniques to be reinforced and bad maneuvers to be corrected. As the trainee progressed from novice, they gained more autonomy and required less instruction and assistance until doing cases independently but with the trainer available. This is consistent with surgical teaching/education theory (22). In a meta-analysis of patients undergoing Colorectal surgery the outcomes of patients undergoing laparoscopic resection were not significantly different between mentored trainees and experienced surgeons (19). Our results show that well supervised training in uniportal VATS lobectomy does not affect patient outcomes and is safe.
Adequacy of lymph node assessment is a marker of oncological quality and surgeons in the early learning curve may dissect less lymph nodes for sampling (27). Lymph node dissection is performed in our unit as per the BTS (14) and ESTS/ERS guidelines (28). There was no difference in number of lymph node stations dissected between consultant scrubbed and unscrubbed groups. The important benefit of supervised VATS training here is that the lymph node dissection is only complete once the trainer is satisfied, whether that be scrubbed or unscrubbed watching the screen.
The most common cause for trainee failure to progress independently was fissure related. This was late in the procedure, 5/8 cases, when the vessels/bronchus had been completed. Part of the reason for this was the need for more advanced camera manipulation during this part of the procedure. VATS training has been recommended to be done with an experienced assistant (29). However here we feel that using a junior assistant speeds up and enhances the learning process so that the trainee has a broader understanding of the operation and has to learn to communicate/manipulate the retraction and camera requirements themselves. Of the 36 cases only two required the trainer to scrub as a second senior assistant.
The main weakness of this paper, like many of the other learning curve articles already written, is that this is the experience of a single trainee/trainer combination. We are now reviewing the curves of further trainee/trainer combinations over the same time period within our unit who use a variety of different VATS approaches to show that the principle of removing case selection and availability of an experienced trainer enhances the training experience whilst achieving good patient outcomes.
Conclusions
Here we have shown that a trainee with a logbook consistent with their level of training and no previous uniportal exposure can achieve short learning curves to become proficient in uniportal VATS lobectomy and segmentectomy with a modified teaching style progressing from a combination of the traditional resident/fellow and proctorship programmes. We feel that traditional methods such as tailored appropriate training lists and use of courses/wetlabs can be used in conjunction with removing case selection to enhance the speed of training under the supervision of an experienced trainer. Video technology is crucial to allow a trainer to be unscrubbed and remain able to teach effectively both during and after cases. Post-operative video critique reinforces learning points repeatedly.
As uniportal VATS becomes more established worldwide the implementation of robust uniportal VATS training programmes will become a priority. Uniportal VATS training, with the correct supervision, can be safe and effective.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kazuo Yoshida) for the series “Robotic VS Uniportal VATS” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/vats-19-62
Data Sharing Statement: Available at http://dx.doi.org/10.21037/vats-19-62
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats-19-62). The series “Robotic VS Uniportal VATS” was commissioned by the editorial office without any funding or sponsorship. DGR serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Jun 2019 to May 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Following internal review, it was decided to waive the need for ethics approval for the collection, analysis and publication of the retrospective anonymized data for this non-interventional study. All patients were diagnosed and treated according to national guidelines. All participants provided written informed consent to use their anonymized data for research purposes prior to the procedure.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive summary: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Petersen RH, Hansen HJ. Learning curve associated with VATS lobectomy. Ann Cardiothorac Surg 2012;1:47-50. [PubMed]
- Bertolaccini L, Batirel H, Brunelli A, et al. Uniportal video-assisted thoracic surgery lobectomy: a consensus report from the Uniportal VATS Interest Group (UVIG) of the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. 2019;56:224-9. [Crossref] [PubMed]
- McKenna RJ Jr. Complications and learning curves for video-assisted thoracic surgery lobectomy. Thorac Surg Clin 2008;18:275-80. [Crossref] [PubMed]
- Liu X, Chen X, Shen Y, et al. Learning curve for uniportal video-assisted thoracoscopic surgery lobectomy-results from 120 consecutive patients. J Thorac Dis 2018;10:5100-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Al-Sahaf M, Lim E. The association between surgical volume, survival and quality of care. J Thorac Dis 2015;7:S152-5. [PubMed]
- Sihoe ADL, Han B, Yang TY, et al. The advent of ultra-high volume thoracic surgical centers in Shanghai. World J Surg 2017;41:2758-68. [Crossref] [PubMed]
- Sihoe ADL, Gonzalez-Rivas D, Yang TY, et al. High-volume intensive training course: a new paradigm for video-assisted thoracoscopic surgery education. Interact Cardiovasc Thorac Surg 2018;27:365-71. [Crossref] [PubMed]
- Hernandez-Arenas LA, Lin L, Purmessur RD, et al. Uniportal video-assisted thoracoscopic early learning curve for major lung resections in a high volume training center. J Thorac Dis 2018;10:S3670-7. [Crossref] [PubMed]
- Billè A, Okiror L, Harrison-Phipps K, et al. Does previous surgical training impact the learning curve in video-assisted thoracic surgery lobectomy for trainees? Thorac Cardiovasc Surg 2016;64:343-7. [PubMed]
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65:iii1-27. [Crossref] [PubMed]
- SAC cardiothoracic surgery, finalising the definitions of a ‘case’. Date: 2015-11-02 08:16.
- Hernandez-Arenas LA, Purmessur RD, Gonzalez-Rivas D. Uniportal video-assisted thoracoscopic segmentectomy. J Thorac Dis 2018;10:S1205-14. [Crossref] [PubMed]
- Hernandez-Arenas LA, Lin L, Yang Y, et al. Initial experience in uniportal subxiphoid video-assisted thoracoscopic surgery for major lung resections. Eur J Cardiothorac Surg 2016;50:1060-6. [Crossref] [PubMed]
- Hernandez-Arenas LA, Guido W, Jiang L. Learning curve and subxiphoid lung resections most common technical issues. J Vis Surg 2016;2:117. [Crossref] [PubMed]
- Miskovic D, Ni M, Wyles SM, et al. Learning curve and case selection in laparoscopic colorectal surgery: systematic review and international multicenter analysis of 4852 cases. Dis Colon Rectum 2012;55:1300-10. [Crossref] [PubMed]
- Divisi D, Bertolaccini L, Barone M, et al. National adoption of video-assisted thoracoscopic surgery (VATS) lobectomy: the Italian VATS register evaluation. J Thorac Dis 2018;10:330-8. [Crossref] [PubMed]
- Yu WS, Lee CY, Lee S, et al. Trainees can safely learn video-assisted thoracic surgery lobectomy despite limited experience in open lobectomy. Korean J Thorac Cardiovasc Surg 2015;48:105-11. [Crossref] [PubMed]
- DaRosa DA, Zwischenberger JB, Meyerson SL, et al. A theory-based model for teaching and assessing residents in the operating room. J Surg Educ 2013;70:24-30. [Crossref] [PubMed]
- Ferguson J, Walker W. Developing a VATS lobectomy programme--can VATS lobectomy be taught? Eur J Cardiothorac Surg 2006;29:806-9. [Crossref] [PubMed]
- Billè A, Okiror L, Karenovics W, et al. Thoracoscopic lobectomy: is a training program feasible with low postoperative morbidity? Gen Thorac Cardiovasc Surg 2013;61:409-13. [Crossref] [PubMed]
- Konge L, Petersen RH, Hansen HJ, et al. No extensive experience in open procedures is needed to learn lobectomy by video-assisted thoracic surgery. Interact Cardiovasc Thorac Surg 2012;15:961-5. [Crossref] [PubMed]
- Okyere S, Attia R, Toufektzian L, et al. Is the learning curve for video-assisted thoracoscopic lobectomy affected by prior experience in open lobectomy? Interact Cardiovasc Thorac Surg 2015;21:108-12. [Crossref] [PubMed]
- Hernandez-Arenas L, Bilancia R, Internullo E. P-170 Mediastinal nodal dissection in the learning curve of video-assisted thoracoscopic anatomic resections: is it good enough? Interact Cardiovasc Thorac Surg 2014;18:S45. [Crossref]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [Crossref] [PubMed]
- Meyerson SL, Balderson SS, D'Amico TA. Training assistants improves the process of adoption of video-assisted thoracic surgery lobectomy. Ann Thorac Surg 2015;100:401-6. [Crossref] [PubMed]
Cite this article as: Mahendran K, Budacan AM, Abiuso V, González-Rivas D, Hernandez-Arenas LA. Optimizing the uniportal video-assisted thoracic surgery learning curve in major lung resection. Video-assist Thorac Surg 2020;5:33.


