
Minimally invasive transhiatal esophagectomy
Introduction
While traditionally performed through an open approach, the role of minimally invasive technologies is evolving in its application to esophageal resection. Esophagectomy is associated with significant morbidity, which has led to interest in developing minimally invasive esophagectomy (e.g. , laparoscopic/thoracoscopic approaches) to address this issue. While a body of literature has grown around minimally-invasive Ivor Lewis and McKeown (3-hole) esophagectomy, publications describing minimally invasive transhiatal esophagectomy (MI-THE) have been less common (1). DePaula and colleagues were the first to describe a laparoscopic approach for transhiatal esophagectomy in 1995 in a series of 12 patients (2). Since this description, groups have also sought to apply robotic technology to facilitate minimally invasive approaches to esophagectomy. As MI-THE has emerged, further evaluation of perioperative and oncologic outcomes, as has been done in the minimally invasive Ivor Lewis and McKeown approaches, will be necessary. Our group has sought to adapt laparoscopic and robotic techniques to the transhiatal approach for both malignant and end-stage benign esophageal disease.
Evolution and practice of robotic transhiatal esophagectomy
Although minimally invasive esophagectomy approaches are well-described in the literature for esophageal malignancies (3,4), the efficacy of robotic-assisted esophagectomy is not as well established. Robotic-assisted esophagectomy was reported initially in 2002 by Melvin and colleagues describing one procedure in a series of other robotic foregut operations (5). Shortly thereafter, additional small series began emerging describing experience with robotic-assisted esophagectomy (6,7). Since the initial reports of this application in 2002–2003, the adoption of this technology for esophagectomy has continued to expand.
Preoperative preparation
After complete cancer staging, patients who are considered fit for major surgery should undergo standard preoperative evaluation. Patients with distal esophageal or esophagogastric junction tumors should undergo routine upper GI endoscopy to ensure that the tumor does not extend into the fundus such that a gastric conduit cannot be prepared with an adequate resection margin. Assessment and subsequent optimization of nutritional status, smoking cessation, and initiation of an exercise program can help patients prepare adequately for an esophagectomy. In addition, patients are oriented to use of the incentive spirometer for preoperative chest physiotherapy.
Pre-incision considerations
After induction of anesthesia, a single lumen endotracheal tube is placed. Large bore IV access and an arterial line should be placed given the risk for hypotension during mediastinal dissection or passing the conduit through the posterior mediastinum. Flexible esophagoscopy is performed to evaluate the esophagus, tumor location, and assessment for the extent of gastric involvement. Once esophagoscopy is completed, a 16 Fr nasogastric tube is placed.
Positioning
The patient is placed in the supine position on the operating room table with the arms tucked at the sides. A shoulder roll is placed and the head is turned to the right. It is important to support the head while ensuring adequate extension of the neck to accentuate the border of the left sternocleidomastoid for the cervical phase of the operation. A footboard is placed in preparation for reverse Trendelenburg positioning to improve exposure during the laparoscopic hiatal dissection.
First abdominal portion
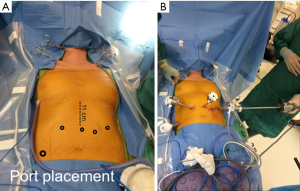
Port placement
Ports are placed as shown (Figure 1A,B) in a configuration for robotic transhiatal esophagectomy. After establishing pneumoperitoneum, intraperitoneal access is obtained and the abdomen is insufflated with CO2 to a pressure of 15 mmHg. For retraction of the left lateral segment of the liver, a Nathanson liver retractor is placed in the subxiphoid area or a paddle liver retractor placed through a 12 mm port in the right lower quadrant. For longer cases, we have found the paddle retractor to cause less trauma to the liver. Additional port placement techniques have been described by other institutions performing robotic-assisted MI-THE (8). The patient is placed in reverse Trendelenburg position. Prior to any dissection, abdominal exploration is completed to inspect for evidence of metastatic disease.
Gastric mobilization
We typically use a bipolar vessel sealing device to mobilize the stomach. Care is taken to avoid trauma to the stomach by minimizing grasping or using a “no-touch” technique using gauze rolls to manipulate the stomach. The right gastroepiploic artery, which constitutes the blood supply to the conduit, is identified and preserved throughout mobilization of the gastric conduit. We typically identify the course of the right gastroepiploic artery and identify the avascular area between where the artery terminates and the short gastric vessels begin. We begin our dissection in this “clear space” and enter the lesser sac and continue to mobilize the greater curvature proximally towards the left crus, dividing the short gastric vessels 1 to 1.5 cm away from the stomach to avoid thermal injury to the stomach.
Once the short gastric vessels are divided, we begin our dissection along the left crus and the anterior aspect of the esophagus, paying attention to avoid denuding the muscle fibers of the hiatus. The omentum is then separated along the greater curvature of the stomach working towards the distal stomach to the level of the pylorus. Ongoing inspection of the course of the right gastroepiploic artery is essential to ensure the omentum is divided 1 to 2 cm inferior to the vessel. The gastrohepatic ligament is divided with the stomach retracted towards the patient’s left. Care is taken to identify a replaced left hepatic artery, which is present in 15% of patients and is preserved. The right crus is identified, and the esophagus is dissected away from the crus, proceeding proximally into the mediastinum (Video 1).
Mobilization of the thoracic esophagus
The esophagus is dissected circumferentially up to the level where the subcarinal lymph nodes are visualized (Video 2). Paraesophageal and subcarinal lymph nodes are dissected from the area and submitted for pathologic analysis. We limit using energy devices for this portion of the nodal dissection in order to avoid inadvertent thermal injury to the posterior membranous airway of the left mainstem bronchus. In addition, it is important not to violate the left or right pleura during the dissection, particularly in patients who have received neoadjuvant radiation therapy. If either pleural space is entered, tube thoracostomy should be considered to avoid hemodynamic compromise from CO2 related pneumothorax. This phase can be deferred until after gastroduodenal mobilization (Kocher maneuver) in order to limit the risk of losing CO2 insufflation before completing most of the abdominal portion of this operation.
Division of the left gastric artery
The stomach is elevated to expose the left gastric pedicle. The celiac lymph nodes are dissected off the vessels to be included in the specimen. The left gastric artery and vein are then separated. The vessels may be ligated with the vessel sealer or with a vascular stapler (Video 3). Care is taken to preserve the common hepatic and splenic arteries.
Kocher maneuver
During the robotic approach, gentle downward traction is placed on the pylorus. In order to maximize length of the conduit, a Kocher maneuver is performed using either the vessel sealer or bipolar device (Video 4). Once the Kocher maneuver is complete, the robotic console is undocked and the midline 8 mm robotic port is converted to a 7 cm utility incision. A wound protector is placed to help with exposure. During the laparoscopic approach, the duodenal mobilization can be performed either laparoscopically or through the midline utility incision. Once the Kocher maneuver is complete the pylorus should be mobilized enough to reach the esophageal hiatus without tension.
Pyloromyotomy
To decrease the risk of gastric outlet obstruction after dividing the vagus nerves, a 2 cm long pyloromyotomy (1.5 cm on the stomach and extending 0.5 cm onto the duodenum) is performed using a fine mosquito clamp and the cutting currently on electrocautery, as has been previously described (9).
Enteral access
A feeding jejunostomy tube is placed using a 14 Fr red rubber catheter 25 cm distal to the Ligament of Treitz. A 4 cm Witzel maneuver is performed. The jejunostomy tube is passed through the abdominal wall later in the procedure.
Cervical neck phase
An incision parallel to the anterior border of the left sternocleidomastoid is performed from the level of the cricoid to the sternal notch. The sternocleidomastoid is retracted laterally, and the omohyoid is divided. The trachea and thyroid are retracted medially using an index finger to avoid trauma to the left recurrent laryngeal nerve. The middle thyroid vein and the inferior thyroid artery are ligated. The esophagus is identified, palpating the nasogastric tube within the esophagus. Sharp dissection is used posterolateral to the tracheoesophageal groove to mobilize the cervical esophagus away from the trachea and left recurrent laryngeal nerve. The cervical esophagus is then encircled with a 1-inch Penrose drain. Upward traction is placed on the Penrose drain as an index finger is used to bluntly dissect the proximal esophagus circumferentially.
Once this is complete, the surgeon’s left hand is placed through the abdominal utility incision and up through the hiatus. Any remaining intrathoracic esophageal mobilization is performed using the standard approach that has been previously described (9). Once the mobilization is complete the cervical esophagus is elevated, the nasogastric tube is withdrawn by the anesthetist above the level of transection, and the cervical esophagus is divided obliquely using a GIA-60 stapler. The nasogastric tube is not removed from the esophagus as its replacement can be difficult after the proximal esophagus has been mobilized for the anastomosis. The specimen is delivered through the abdominal incision. A sump catheter is introduced into the posterior mediastinum through the cervical incision to the level of the hiatus to assist in identifying any significant intrathoracic bleeding. The superior mediastinum is gently packed through the cervical incision for hemostasis. The mediastinum is also examined through the abdominal incision with a Deaver retractor in the hiatus to confirm hemostasis and any openings in the pleura. Chest tubes are placed at this time as needed, and the mediastinum is packed with a laparotomy sponge.
Second abdominal phase
Creation of the gastric conduit
After dividing 2–3 vessels along the lesser curve, the gastric conduit is created using multiple firings of a 3.8 mm GIA-60 stapler to “unroll” the stomach, as previously described (9). Following creation of the conduit, we routinely assess the vascularization of the conduit using indocyanine-green dye and near infrared fluorescence imaging. If there are any concerns with perfusion of the most distal aspect of the gastric conduit, revision of this site can be performed, even after advanced to the neck. The gastric conduit is introduced through the hiatus and advanced through the posterior mediastinum. A Babcock clamp can be applied from the cervical incision in order to guide the conduit through the thoracic inlet and delivered to the cervical incision. Care is taken during passage of the conduit to avoid traumatizing the stomach by pushing, as opposed to pulling, the conduit up. It is important to ensure that the conduit is oriented correctly with the staple line positioned towards the patient’s right side.
During the robotic approach, a hand port is then placed and the abdomen reinsufflated. The robot is re-docked and the surgeon’s hand is placed through the hand port to inspect the size of the hiatus. The hiatus is closed anteriorly to 2–3 fingerbreadths using number 1 silk sutures using the large suture-cut needle driver (Video 5). With the laparoscopic approach, the hiatus can either be closed through the utility incision or laparoscopically. One or two 3-0 silk sutures may be placed between the gastric wall and the hiatus to help prevent herniation through the hiatus.
The jejunostomy tube is passed through the left upper quadrant port site and sutured in place. The abdominal incision and associated port sites are then closed.
Second neck phase
Cervical esophagogastric anastomosis
A side-to-side semi-stapled cervical esophagogastric anastomosis is performed as previously described (9). A purple load EndoGIA stapler is used for the back wall of the anastomosis. After gently guiding a 16 Fr nasogastric tube through the anastomosis, a running inner layer and interrupted Lembert 4-0 PDS sutures are used to complete the front wall of the anastomosis. A Penrose drain is placed.
Postoperative care
A chest X-ray is performed in the operating room prior to awakening from anesthesia to ensure that there are no unrecognized pneumothoraces and that the nasogastric tube is properly positioned within the gastric conduit. The nasogastric tube is typically removed on postoperative day 3. A barium esophagogram is performed on postoperative day 6 or 7.
Perioperative and oncologic outcomes
When evaluating MI-THE specific approaches, a systematic review focusing on robotic esophagectomy detailed previous experience with robotic-assisted transhiatal esophagectomy (10). Operative time was generally shorter compared to transthoracic approaches and ranged from 231 to 312 minutes. Major morbidity rates ranged from 23% to 32% across studies included in the systematic review. Operative times for the transthoracic approach ranged from 367 to 693 minutes. Mortality was found to range from 0% to 6% in this study, although mortality rates were limited across studies detailing the transhiatal approach.
A report from van der Horst and colleagues focusing on 31 patients with malignancy of the upper esophagus observed an in-hospital mortality rate of 10% (11). This highlights some of the limitations of this approach for specific types of esophageal lesions. It is also important to consider the substantial learning curve with complex minimally invasive or robotic procedures. However, other retrospective studies have demonstrated perioperative outcomes similar to open esophagectomy suggesting that robotic-assisted MI-THE can be safely performed (12,13). There are limited data for comparison between robotic-assisted MI-THE and the laparoscopic approach. However, findings from a small retrospective study demonstrated similar results between both approaches.
With increasing experience in robotic-assisted esophagectomy, data evaluating outcomes to guide patient selection (i.e. , BMI, neoadjuvant therapy, advanced age) for this procedure have emerged as well (14-16). While not specific to the type of esophagectomy performed, this highlights that robotic assistance may have a specific role for certain patient populations, such as those with an elevated BMI.
Conclusions
As experience continues to develop with MI-THE, future studies will continue to evaluate not only perioperative and oncologic outcomes, but also cost efficiency. Special consideration will be needed to evaluate surgeon “learning curve” associated with MIE-THE while applying novel techniques to an already complex procedure.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Robert E. Merritt) for the series “Minimally Invasive Esophagectomy for Esophageal Carcinoma” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/vats-2019-mie-03). The series “Minimally Invasive Esophagectomy for Esophageal Carcinoma” was commissioned by the editorial office without any funding or sponsorship. RMR serves as an unpaid editorial board member of in Video-Assisted Thoracic Surgery from Mar 2020 to Mar 2022. Dr. RMR reports personal fees from Intuitive Surgical, personal fees from Auris Health, personal fees from Medtronic, outside the submitted work. Dr. RMR is a robotic proctor for Intuitive Surgical, Inc. , an advisor for Medtronic, and a consultant for Auris Surgical. Dr. JL reports financial disclosures from Intuitive Surgical, Inc, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Battafarano RJ. Minimally Invasive Esophagectomy: Is There an Advantage? Adv Surg 2016;50:17-28. [Crossref] [PubMed]
- DePaula AL, Hashiba K, Ferreira EA, et al. Laparoscopic transhiatal esophagectomy with esophagogastroplasty. Surg Laparosc Endosc 1995;5:1-5. [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Yerokun BA, Sun Z, Yang CJ, et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Population-Based Analysis. Ann Thorac Surg 2016;102:416-23. [Crossref] [PubMed]
- Melvin WS, Needleman BJ, Krause KR, et al. Computer-enhanced robotic telesurgery. Initial experience in foregut surgery. Surg Endosc 2002;16:1790-2. [Crossref] [PubMed]
- Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. [Crossref] [PubMed]
- Horgan S, Berger RA, Elli EF, et al. Robotic-assisted minimally invasive transhiatal esophagectomy. Am Surg 2003;69:624-6. [PubMed]
- Gutt CN, Bintintan VV, Koninger J, et al. Robotic-assisted transhiatal esophagectomy. Langenbecks Arch Surg 2006;391:428-34. [Crossref] [PubMed]
- Orringer MB. Transhiatal Esophagectomy: How I Teach It. Ann Thorac Surg 2016;102:1432-7. [Crossref] [PubMed]
- Ruurda JP, van der Sluis PC, van der Horst S, et al. Robot-assisted minimally invasive esophagectomy for esophageal cancer: A systematic review. J Surg Oncol 2015;112:257-65. [Crossref] [PubMed]
- van der Horst S, Weijs TJ, Ruurda JP, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy for esophageal cancer in the upper mediastinum. J Thorac Dis 2017;9:S834-S842. [Crossref] [PubMed]
- Coker AM, Barajas-Gamboa JS, Cheverie J, et al. Outcomes of robotic-assisted transhiatal esophagectomy for esophageal cancer after neoadjuvant chemoradiation. J Laparoendosc Adv Surg Tech A 2014;24:89-94. [Crossref] [PubMed]
- Dunn DH, Johnson EM, Anderson CA, et al. Operative and survival outcomes in a series of 100 consecutive cases of robot-assisted transhiatal esophagectomies. Dis Esophagus 2017;30:1-7. [Crossref] [PubMed]
- Shridhar R, Abbott AM, Doepker M, et al. Perioperative outcomes associated with robotic Ivor Lewis esophagectomy in patient's undergoing neoadjuvant chemoradiotherapy. J Gastrointest Oncol 2016;7:206-12. [PubMed]
- Salem AI, Thau MR, Strom TJ, et al. Effect of body mass index on operative outcome after robotic-assisted Ivor-Lewis esophagectomy: retrospective analysis of 129 cases at a single high-volume tertiary care center. Dis Esophagus 2017;30:1-7. [PubMed]
- Abbott A, Shridhar R, Hoffe S, et al. Robotic assisted Ivor Lewis esophagectomy in the elderly patient. J Gastrointest Oncol 2015;6:31-8. [PubMed]
Cite this article as: Grenda TR, Lin J, Chang AC, Reddy RM. Minimally invasive transhiatal esophagectomy. Video-assist Thorac Surg 2021;6:8.


