
Personalized approach for video-assisted thoracic surgery lung metastasectomy
Introduction
Pulmonary metastases are diagnosed in 30% of patients with solid malignant tumors and are known to worsen their prognosis (1,2). Surgery has been demonstrated to allow improvement of survival prognosis, if several selection criteria are met (1,3). As a result, surgical pulmonary metastasectomy (PM) has become an accepted option for the multimodal management of these patients and an important part of thoracic surgeons’ daily activities (4-7).
Since the introduction of the video-assisted thoracic surgery (VATS) technique in the early 1990s, several series demonstrated its advantages over open thoracotomy for PM (8-11). In brief, VATS allows a better visualization of the pleural cavity, generates less post-operative pain and morbidity, thus shorter hospital stays as compared to thoracotomy (12). Moreover, fewer adhesions are created thanks to smaller incisions and less traumatic tissue dissection. This facilitates repeated surgeries in case of the frequently observed recurrences (12). The importance of the VATS approach is even higher with the recent development of thin-slice (1-mm) CT-scans, which allow a better detection of pulmonary metastases before surgery (8). Thus, complete resection of pulmonary metastases can be achieved by VATS in most cases, since the majority of pulmonary metastases are peripherally located and easily accessible.
However, various criteria for choosing a VATS approach over open thoracotomy for PM are described in the literature. In addition, authors tend to emphasize different elements, which creates an unclear situation when it comes to decision-making criteria for the more suitable approach. Ideally, these would be streamlined and generally accepted. Since 2003, we have adopted a VATS approach for solitary pulmonary metastasis when technically feasible. However, with growing experience, we have extended our criteria for multiple peripheral lesions or centrally located lesions requiring an anatomical pulmonary resection. Our decision-making process also includes a multidisciplinary discussion of each case in regular tumor board meetings and the surgical approach as well as parenchymal resection type and extent are individually decided based on several criteria (Table 1) (13,14).

Full table
We analyzed our decision criteria to ensure that we did not only offer VATS approach to the most eligible patients, but that we achieved the highest possible benefits for a given patient by choosing the absolutely best surgical approach for each individual case. Our study objective was to analyze the postoperative outcomes of patients undergoing VATS PM and to determine prognostic factors of survival and recurrence in order to propose a personalized approach for VATS PM. We present the following article in accordance with the STROBE Reporting Checklist (available at http://dx.doi.org/10.21037/vats-2020-lm-04).
Methods
Study design
This study includes a review of the data of all consecutive adult patients who underwent PM by VATS in our institution between July 2003 and November 2018. We excluded patients who underwent a diagnostic procedure, an open thoracotomy or whose operation had to be converted from VATS to open thoracotomy during surgery.
Patient selection
Each individual case was discussed in an interdisciplinary tumor board involving oncologists, thoracic surgeons, pulmonologists and radiologists. Our personalized treatment strategy was defined to spare pulmonary parenchyma to the maximal possible extent whilst offering the least invasive procedure.
First, within one month prior to surgery, all patients underwent a complete radiological and physiological assessment, including a thin-slice (1 mm) helical chest CT-scan to precisely determine the number, size and localization of pulmonary metastases and a positron emission tomography (PET) scan to evaluate lymph node (LN) involvement and exclude extra-thoracic metastases if primary tumor was avid on fluorodeoxyglucose (FDG). In case of unclear diagnosis, the results of a trans-bronchial or percutaneous biopsy were added. Each patient’s tolerance to surgical resection was tested by spirometry and transthoracic echocardiography (15).
A surgical PM indication was agreed upon for carefully selected patients who fulfilled the following criteria (3): (I) the pulmonary metastases can be completely resected; (II) the primary tumor is controlled; (III) there is no other extra-pulmonary metastases or if there are, they can be resected completely before PM; (IV) the pulmonary metastases can be completely resected without impacting the patient’s respiratory functions; (V) there is no better alternative systemic treatment.
The extent and type of pulmonary resection was individually discussed based on the localization, size and number of metastases. We defined an indication for VATS when there were three lesions or fewer, all lesions were peripherally located and amenable to wedge-resection or, in cases of central lesions, segmentectomy or lobectomy were possible. In selected cases, more than three lesions could be potentially resected by VATS when peripherally located or if all lesions were present in the lobe or segment requiring an anatomical resection. Additionally, the size of the lesion was determinant if it could be removed through the utility incision without rib retractor (normally less than 5 cm). When anatomical resection was required by VATS but lesser resection was possible by thoracotomy, thoracotomy was chosen based on our policy to spare pulmonary parenchyma to the maximal possible extent.
Data collection
We extracted data from our electronic database and medical records on a retrospective basis. Data included: patients demographics and comorbidities; primary tumour and pulmonary metastasis characteristics; surgical characteristics; post-operative outcomes up to 30 days after surgery; recurrences and repeated PM (RPM), if any, and their characteristics. In addition, disease-free interval (DFI) and overall survival (OS) were assessed. The time interval between the resection of the primary tumour and the first PM was defined as DFI1 and the time interval between first PM and cancer recurrence was defined as DFI2. Pulmonary metastases were defined as “synchronous” when diagnosed at the same time as the primary tumour diagnosis and “metachronous” otherwise.
Operation and follow-up
Surgery was performed under general anaesthesia with protective single-lung ventilation. If necessary, for small and deep-located metastases, a percutaneous hook wire device was placed pre-operatively under CT-scan guidance to facilitate the intra-operative detection of the lesion. The VATS approach consisted in a classical three-port anterior approach, with an anterior 3-cm mini-thoracotomy in the fifth intercostal space, an anterior 10-mm trocar for the camera and a posterior 5-mm working incision. Mediastinal LN dissection was realized for lesions requiring an anatomical resection or when LN involvement was suspected on pre-operative radiological exams. Pulmonary specimens were extracted in a protective bag through the mini-thoracotomy. If the surgeon had doubts about the completeness of resection, a histological frozen section was performed. After surgery, all patients were transferred to the ward, except in case of severe cardiopulmonary comorbidities requiring active monitoring. The need for adjuvant therapy was discussed in the interdisciplinary tumour board for each patient. The follow-up consisted in a thoraco-abdominal CT-scan at 3, 6, 12, 18 and 24 months and then on a yearly basis.
Statistical analysis
The results are presented as numbers with percentages for binary variables and medians with interquartile range (IQR) or means with standard deviation (SD) for continuous variables. OS was calculated using the Kaplan-Meier and log-rank analyses. Cox regression univariate analysis was performed to identify prognostic factors of recurrence and survival. A two-tailed hypothesis was used and significance accepted if P<0.05. The Stata version 14 software (StataCorp, Texas USA) was used for statistical analyses. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by local ethics committee (No. 2019-02474) and individual consent for this retrospective analysis was waived.
Results
A total of 189 patients (female/male: 84/105) with a median age of 64 years (IQR 52–71 years) underwent PM by VATS in the context of colorectal cancer (30.2%), melanoma (20.1%), sarcoma (14.3%) and other primary tumours (35.4%). Patient characteristics are shown in Table 2. The median DFI1 was 31 months (IQR 15–58 months) and 23 (12.2%) patients presented synchronous metastases. Pulmonary metastases were solitary in 77.2% and multiple (range, 2–8) in 22.8% of cases. They were unilateral in 84.1% of cases. Wedge resection was performed in 156 (82.5%), segmentectomy in 15 (7.9%) and lobectomy in 18 (9.5%) patients. Complete resection was achieved in 97.9% of cases and only four patients (2.1%) presented an R1 status, with tumoral invasion of the section slice. Mediastinal LNs dissection was realized in 29 (15.3%) and LN involvement was diagnosed in 5 (2.6%) patients.

Full table
There was no 30-day mortality and the overall 30-day morbidity rate was 7.9%, with 5.8% of pulmonary and 1.1% of cardiac complications. The median durations of drainage and hospital stay were 1 day (IQR 1–2 day) and 3 days (IQR 3–5 day), respectively. Three patients (1.6%) were readmitted during 30-day post-operative course. The first two required pleural drainage because of an inflammatory pleural effusion and a pneumothorax, without further complication. The third patient underwent a complementary resection by VATS because of an R1 status.
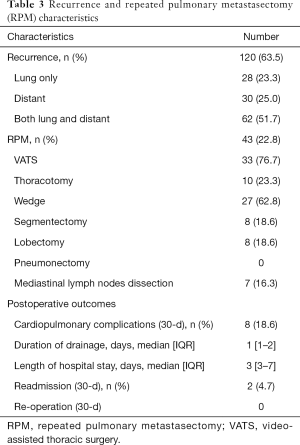
During the follow-up (median 33 months; IQR 17–56 months), 120 patients (63.5%) presented a recurrence, with 28 (23.3%) patients with a recurrence in the lung only, 30 (25%) with a distant recurrence and 62 (51.7%) with both lung and distant recurrences (Table 3). The median DFI2 was 8 months (IQR 4–16.8 months). Of those 120 patients, only 43 (35.8%) underwent an RPM which involved the ipsilateral lung in 16 cases (37.2%). The RPMs were performed by VATS in 33 cases (76.7%) or by thoracotomy in 10 cases (23.3%). The extent of resection for the RPM procedures consisted in: wedge in 27 (62.8%), segmentectomy in 8 (18.6%) and lobectomy in 8 patients (18.6%). The 30-day post-operative cardiopulmonary complication rate was 18.6%. Two patients (4.7%) were readmitted for inflammatory pleural effusion requiring drainage without further complication. There was no re-operation after RPM.
Full table
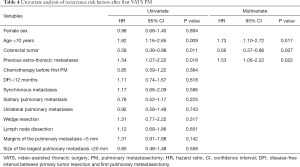
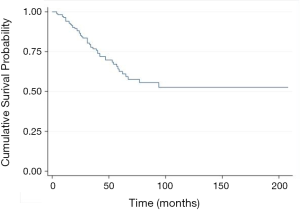
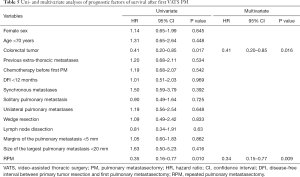
Multivariate analyses identified two risk factors of recurrence: age <70 years (HR 1.73, 95% CI, 1.10–2.72, P=0.017), previous extra-thoracic metastases (HR 1.53, 95% CI, 1.06–2.22, P=0.022); and one protective factor against recurrence: colorectal cancer (HR 0.56, 95% CI, 0.37–0.86, P=0.007) (Table 4). The 5-year OS rate after first PM was 63% (Figure 1). Two prognostic factors of better survival were identified in the multivariate analyses: colorectal cancer (HR 0.41, 95% CI, 0.20–0.85, P=0.016) and RPM (HR 0.34, 95% CI, 0.15–0.77, P=0.009) (Table 5).
Full table
Full table
Discussion
Over time, the predominant surgical approach for PM shifted from traditional open thoracotomy to VATS. This shift occurred in parallel to technological developments. Yet, a consensus over which approach is preferable is lacking, as are randomized trials analysing outcomes. Published retrospective studies and meta-analyses showed that VATS approach is comparable to open surgery in terms of survival and local recurrence (12,16-18). Then, either approach has been used based on surgeons’ preference and on the localization and size of the metastases. A number of surgeons embrace the advantages of VATS such as reducing surgical trauma, enhancing post-operative recovery, and minimizing pleural adhesions. This last advantage is particularly relevant considering the elevated probability of recurrence and future repeated resections (10,12,19). However, other surgeons favour an open approach to facilitate identification of all targeted lesions via bimanual palpation, thus minimizing the risk to overlook deep and centrally located metastases (20). In this context, and based on an agreed consensus that the treatment of metastases must be discussed on a case-by-case basis within a multidisciplinary team, the aim of the present study was to analyse the post-operative outcomes and prognostic factor of recurrence and survival of patients after individual discussion for VATS PM. Whereas we understand that in some situations, more lung parenchyma can be spared during open surgery (i.e., precision surgery or laser resections), some authors advocate an open thoracotomy for optimal identification of occult metastases that may have been missed by pre-operative CT scan. In cases where parenchyma can be saved with precision surgery by thoracotomy but not feasible by VATS without anatomical resection, we prefer to propose an open approach. With that exception in mind, in our opinion, the necessity to carry out bimanual palpation, thus to advocate open surgery, dwindled over the past few years as a result of two developments: the advances in radiological imaging with the routine use of 1-mm thin-slice CT-scan, and the improvement of dedicated VATS instrumentation and perioperative localization (hook wire, coils, etc.). As a result, we found that bimanual palpation lost some significance in the current context of very careful selection of patients. Nowadays, it is possible to offer an optimal treatment and to avoid thoracotomy and bimanual palpation in those patients for whom these procedures might be unnecessarily invasive and offer little added value. This seems supported by the Spanish prospective registry from 2016 that analysed the agreement between radiological imaging and the pathological findings of PM in the setting of colorectal cancer patients. Solitary pulmonary nodules were found in 73% of the cases who underwent thoracotomy with bimanual palpation. The radiological and pathological agreement was found to be 95% (21), suggesting that VATS should be offered for patients with solitary lesion with low risk of missing additional nodules.
The number of metastases is often chosen as a decisive factor to determine the surgical approach (VATS or thoracotomy). Our series of 189 patients reveals that 72.5% of patients undergoing VATS PM presented a solitary metastasis. Actually, pulmonary metastases are often peripheral and small-sized, hence easy to remove by VATS wedge resection. This practice is currently largely performed in daily practice according to a survey conducted in Great Britain and Ireland where VATS approach was preferred for 85% of cardio-thoracic surgeon for solitary PM (4). In this sense, the current Society of Thoracic Surgery (STS) expert consensus (22) advocates that minimally invasive surgery can be considered the preferred option for PM because of shortened post-operative recovery and lower effect on quality of life for solitary lesions. However, VATS remains controversial for multiple lesions. We could propose multiple metastasectomies in 22.8%, with 35 patients (18.5%) presenting two or three metastases and eight patients (4.2%) presenting four or more metastases. Hence, the number of metastases per se cannot be considered a formal contraindication as long as complete resection can be achieved without compromising pulmonary functions. Multiple metastasectomy was generally offered for peripheral lesions easily visualised during VATS. In some cases, we experienced situations where multiple lesions were located in one lobe and the only option was the removal of the entire lobe.
Lo Faso et al. (23) reported 212 VATS PM in 164 patients where multiple metastases were present in 41% of patients. Likewise, the series by Nakajima et al. (16) that compared VATS versus open surgery showed that 52% of patients presented multiple metastases. Among those patients, VATS was performed in 21% of the cases. Those results are aligned with our series and demonstrate that multiple metastases are very often managed by open approach although, as mentioned above, this decision should not be solely based on the number of metastases. In addition, the recent series of 211 patients by Prenafeta et al. (18) that compared the ipsilateral recurrence after VATS or open surgery of colorectal lung metastases, showed that the number of metastasis was 1.16 for VATS group and 2.54 for open group. Interestingly, after propensity score matching, no significant difference was observed in ipsilateral recurrence rates between VATS and open surgery. In this sense, we promote the open approach for parenchyma sparing strategies (i.e., precision surgery) in cases of deep located multiple metastases (24).
The intra-operative localisation of the lesion is an additional problem to face during VATS. The difficulty to locate a pulmonary nodule may counterbalance the real benefit of VATS in terms of post-operative outcome. We previously reported our experience with pre-operative computed tomography-guided hook wire localization of solitary nodules with a success rate of 98.3% (25). The diagnostic yield we achieved is comparable to that found in the recent literature (26-28). In this context, the convenience of pre-operative localization by means of a hook-wire is individually decided based on the size and the depth of the nodule. As a rule, we routinely recommend pre-operative localization for small lesions of less than 10 mm in diameter, located at a distance of more than 5mm from the pleural surface due to associated high rate conversion thoracotomy (29).
In our series, 82.5% of patients were treated by means of a wedge resection. This preference for non-anatomical resection might explain the relatively low morbidity rate, with only 7.9% of patients suffering complications during the 30-day post-operative course and no overall 30-day mortality rate (0%). These results are consistent with other surgical series about pulmonary metastases (30,31). In addition, anatomical resections were only performed for centrally located or larger (>5 cm) lesions in order to achieve complete resection. In our series, the type of surgical resection was neither a survival nor a recurrence prognostic factor. For this reason, we believe that major lung resections may be justified in some specific patients. In any case, we followed our guideline of sparing as much healthy parenchyma as possible, provided that free margins could be obtained. Interestingly, a prospective multicentre study that analysed the role of major resection in PM for colorectal cancer showed that major resection was a protective factor that increased the survival rate in comparison with wedge resection (55 vs. 28 months) (32).
Lo Faso et al. (23) reported on a single-centre series of 164 patients who underwent 212 VATS PM from 2000 to 2011. They performed 133 wedge resections, 22 segmentectomies, 54 lobectomies and 1 pneumonectomy. It is noteworthy that although there was no difference in long-term survival among patients who underwent wedge resections or anatomical lung resections, the rate of lobectomies in this series was high compared to other recent (18) or classic open surgery series (33). This is probably explained by the fact that some deep-located nodules could be more easily resected by a VATS lobectomy than by a wedge resection or a complex segmentectomy, which could only be performed by open thoracotomy. However, in our opinion sparing lung parenchyma should prevail over the operative approach. Following this principle, we achieved a rate of major pulmonary resections of 17%. Therefore, we consider that a conversion to thoracotomy should be viewed as an option, based on the informed decision of an experienced surgeon, in order to avoid a more extensive lung resection (12).
Of all analysed risk factors for recurrence after first VATS PM, age <70 years and previous extra-thoracic metastases turned out to be significant, while primary colorectal cancer proved to be a protective factor. In this sense, the younger age was already identified as a risk factor in the classic series by Onaitis et al. (34). It seems reasonable to think that young patients, who in principle have a longer life expectancy, are treated more extensively in the setting of an aggressive primary tumor, and thus have more time to develop recurrences. This may explain why the presence of prior extra-thoracic metastases is also reported to be a risk factor for recurrence. Accordingly, it is a fair assumption that the biology of the tumor plays a role on the progression of the disease, so that colorectal tumors behave less adversely than non-colorectal ones such as sarcomas or melanomas (1,35). Besides, it is noteworthy that during the median follow-up of 33 months after first PM, 63.5% of the patients presented a recurrence as follows: pulmonary only (23.3%), distant (25%) and both pulmonary and distant (51.7%). Some authors advocate an open approach for PM on the assumption that VATS PM would leave some non-palpable nodules unnoticed, so the rate of lung recurrence would be higher. However, published case-matched studies comparing VATS approach versus thoracotomy showed similar ipsilateral pulmonary recurrence rates between procedures as follows: 14.3–28.7% by open approach and 20–25.3% by VATS (9,18).
Among patients who developed recurrences, RPM was performed in 35.8% of the cases. Our data with regards to recurrence rate and RPM is aligned with several published studies (1,9,36-38). The 30-day postoperative cardiopulmonary complication rate was statistically higher after RPM than after first PM (18.6% vs. 7.9%, respectively; P=0.03). Menna et al. reported a post-operative morbidity rate of 11.3% after RPM and 18.3% after first PM, with no statistically significant difference (31). Additionally, Kondo et al. showed that VATS had advantages over thoracotomy as it reduced pulmonary adhesion to parietal pleura at the time of reoperation, shortened the operating time and reduced intraoperative bleeding and duration of thoracic drainage (11). Finally, we performed VATS PM over the past 20 years and we observed that RPM procedures are better accepted by both patients and referent physicians when the first PM had been performed by VATS, probably because of its low morbidity rate.
Our results showed a 5-year OS rate of 63%, consistent with data from literature (16-18). Multivariate analysis showed that primary colorectal tumour (HR 0.41, 95% CI, 0.20–0.85, P=0.02) and RPM (HR 0.35, 95% CI, 0.16–0.77, P=0.01) were prognostic factors for prolonged survival, a fact consistent with values reported by other groups (37-39). It should be noted that the primary tumour histology has been identified as a prognostic factor in many studies (1,2,40,41). In particular, the International Registry of Lung Metastases reported a better 5-year survival rate for patients with germ cell tumours (68%), followed by epithelial tumours (37%), sarcomas (31%) and melanoma (21%).
We generally reserved radical LN dissection for patients with large or central lesions requiring anatomic pulmonary resection (16.3%) or in cases of pre-operative suspicions. Again, as patients with more than three metastases were mainly operated by an open approach, almost all lymphadenectomies were performed on patients who underwent an anatomical resection. However, according to the recent STS expert consensus, LN sampling/dissection concomitant with PM should be considered, because pulmonary metastases accompanied by mediastinal LN metastases predict poor survival (22). In our series, we did not observe advantages in term of survival or recurrence when LN dissection was carried out.
We recognize that there are potential limitations to our study, the first being a retrospective single-centre design. In addition, patients were included over a 15-year period, thus flattening the prognostic impact of the evolving systemic therapies and imaging systems.
In conclusion, authors support the idea that the best approach for PM is the personalized approach to achieve surgical and oncological success (24). Patients’ respiratory and oncological characteristics, the surgical technique, the extent of the resection, the type of primary tumour, the size, localization and number of pulmonary metastases, the resection margins and the LNs management are crucial elements that determine the possibility of a VATS approach. In our opinion, OS, together with recurrence rate, were key points in assessing the effectiveness of a surgical approach. Thus, we demonstrated that performing VATS PM with a personalized approach is safe and favourable in many respects.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Video-Assisted Thoracic Surgery for the series “VATS in Lung Metastasectomy”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist, available at http://dx.doi.org/10.21037/vats-2020-lm-04
Data Sharing Statement: Available at http://dx.doi.org/10.21037/vats-2020-lm-04
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats-2020-lm-04). The series “VATS in Lung Metastasectomy” was commissioned by the editorial office without any funding or sponsorship. MG served as an unpaid Guest Editor of the series. MM served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Jun 2019 to May 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by regional ethics committee (No. 2019-02474) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Pfannschmidt J, Egerer G, Bischof M, et al. Surgical Intervention for Pulmonary Metastases. Dtsch Arztebl Int 2012;109:645-51. [PubMed]
- Thomford NR, Woolner LB, Clagett OT. The Surgical treatment of metastatic tumors in the lungs. J Thorac Cardiovasc Surg 1965;49:357-63. [Crossref] [PubMed]
- Jegatheeswaran S, Satyadas T, Sheen AJ, et al. Thoracic surgical management of colorectal lung metastases: A questionnaire survey of members of the society for cardiothoracic surgery in great britain and ireland. Ann R Coll Surg Engl 2013;95:140-3. [Crossref] [PubMed]
- Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: A systematic review and meta-analysis. Ann Surg Oncol 2013;20:572-9. [Crossref] [PubMed]
- Gonzalez M, Gervaz P. Risk factors for survival after lung metastasectomy in colorectal cancer patients: Systematic review and meta-analysis. Future Oncol 2015;11:31-3. [Crossref] [PubMed]
- Zellweger M, Abdelnour-Berchtold E, Krueger T, et al. Surgical treatment of pulmonary metastasis in colorectal cancer patients: Current practice and results. Crit Rev Oncol Hematol 2018;127:105-16. [Crossref] [PubMed]
- Abdelnour-Berchtold E, Perentes JY, Ris HB, et al. Survival and Local Recurrence after Video-Assisted Thoracoscopic Lung Metastasectomy. World J Surg 2016;40:373-9. [Crossref] [PubMed]
- Chao YK, Chang HC, Wu YC, et al. Management of lung metastases from colorectal cancer: Video-assisted thoracoscopic surgery versus thoracotomya case-matched study. Thorac Cardiovasc Surg 2012;60:398-404. [Crossref] [PubMed]
- Meng D, Fu L, Wang L, et al. Video-assisted thoracoscopic surgery versus open thoracotomy in pulmonary metastasectomy: a meta-analysis of observational studies. Interact Cardiovasc Thorac Surg 2016;22:200-6. [Crossref] [PubMed]
- Kondo R, Hamanaka K, Kawakami S, et al. Benefits of video-assisted thoracic surgery for repeated pulmonary metastasectomy. Gen Thorac Cardiovasc Surg 2010;58:516-23. [Crossref] [PubMed]
- Perentes JY, Krueger T, Lovis A, et al. Thoracoscopic resection of pulmonary metastasis: Current practice and results. Crit Rev Oncol Hematol 2015;95:105-13. [Crossref] [PubMed]
- Perentes JY, Zellweger M, Gonzalez M. Personalized surgery for the management of pulmonary metastasis. J Thorac Dis 2018;10:52-5. [Crossref] [PubMed]
- Gonzalez M, Zellweger M, Nardini M, et al. Precision surgery in lung metastasectomy. Future Oncol 2020;16:7-13. [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic Evaluation of the Patient With Lung Cancer Being Considered for Resectional Surgery: Diagnosis and Management of Lung Cancer, 3rd Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013;143:e166S-e190S.
- Nakajima J, Takamoto S, Tanaka M, et al. Thoracoscopic surgery and conventional open thoracotomy in metastatic lung cancer. Surg Endosc 2001;15:849-53. [Crossref] [PubMed]
- Carballo M, Maish MS, Jaroszewski DE, et al. Video-assisted thoracic surgery (VATS) as a safe alternative for the resection of pulmonary metastases: a retrospective cohort study. J Cardiothorac Surg 2009;4:13. [Crossref] [PubMed]
- Prenafeta Claramunt N, Hwang D, de Perrot M, et al. Incidence of Ipsilateral Side Recurrence After Open or Video-Assisted Thoracic Surgery Resection of Colorectal Lung Metastases. Ann Thorac Surg 2020;109:1591-7. [Crossref] [PubMed]
- Gossot D, Radu C, Girard P, et al. Resection of Pulmonary Metastases From Sarcoma: Can Some Patients Benefit From a Less Invasive Approach? Ann Thorac Surg 2009;87:238-43. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, McCarty TP, et al. A Prospective Study to Determine the Incidence of Non-Imaged Malignant Pulmonary Nodules in Patients Who Undergo Metastasectomy by Thoracotomy With Lung Palpation. Ann Thorac Surg 2011;91:1696-700; discussion 1700-1.
- Marron MC, Lora D, Gamez P, et al. Agreement between computed tomography and pathologic nodule counts in colorectal lung metastases. Ann Thorac Surg 2016;101:259-65. [Crossref] [PubMed]
- Handy JR, Bremner RM, Crocenzi TS, et al. Expert Consensus Document on Pulmonary Metastasectomy. Ann Thorac Surg 2019;107:631-49. [Crossref] [PubMed]
- Lo Faso F, Solaini L, Lembo R, et al. Thoracoscopic lung metastasectomies: A 10-year, single-center experience. Surg Endosc 2013;27:1938-44. [Crossref] [PubMed]
- Perentes JY, Zellweger M, Gonzalez M. Change of paradigm in pulmonary metastasectomy. Ann Thorac Surg 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Hanauer M, Perentes JY, Krueger T, et al. Pre-operative localization of solitary pulmonary nodules with computed tomography-guided hook wire: Report of 181 patients. J Cardiothorac Surg 2016;11:5. [Crossref] [PubMed]
- Park CH, Han K, Hur J, et al. Comparative Effectiveness and Safety of Preoperative Lung Localization for Pulmonary Nodules: A Systematic Review and Meta-analysis. Chest 2017;151:316-28. [Crossref] [PubMed]
- Zhao G, Yu X, Chen W, et al. Computed tomography-guided preoperative semi-rigid hook-wire localization of small pulmonary nodules: 74 cases report. J Cardiothorac Surg 2019;14:149. [Crossref] [PubMed]
- McDermott S, Fintelmann FJ, Bierhals AJ, et al. Image-guided preoperative localization of pulmonary nodules for video-assisted and robotically assisted surgery. Radiographics 2019;39:1264-79. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: Indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Rodríguez-Fuster A, Belda-sanchis J, Aguiló R, et al. Morbidity and mortality in a large series of surgical patients with pulmonary metastases of colorectal carcinoma: A prospective multicentre spanish study (GECMP-CCR-SEPAR). Eur J Cardiothorac Surg 2014;45:671-6. [Crossref] [PubMed]
- Menna C, Berardi G, Tierno SM, et al. Do Repeated Operations for Recurrent Colorectal Lung Metastases Result in Improved Survival? Ann Thorac Surg 2018;106:421-7. [Crossref] [PubMed]
- Hernández J, Molins L, Fibla JJ, et al. Role of major resection in pulmonary metastasectomy for colorectal cancer in the Spanish prospective multicenter study (GECMP-CCR). Ann Oncol 2016;27:850-5. [Crossref] [PubMed]
- Rolle A, Pereszlenyi A, Koch R, et al. Is surgery for multiple lung metastases reasonable? A total of 328 consecutive patients with multiple-laser metastasectomies with a new 1318-nm Nd:YAG laser. J Thorac Cardiovasc Surg 2006;131:1236-42. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Haney JC, et al. Prognostic Factors for Recurrence After Pulmonary Resection of Colorectal Cancer Metastases. Ann Thorac Surg 2009;87:1684-8. [Crossref] [PubMed]
- Migliore M, Milošević M, Lees B, et al. Finding the evidence for pulmonary metastasectomy in colorectal cancer: the PulMicc trial. Future Oncol 2015;11:15-8. [Crossref] [PubMed]
- Blackmon SH, Stephens EH, Correa AM, et al. Predictors of recurrent pulmonary metastases and survival after pulmonary metastasectomy for colorectal cancer. Ann Thorac Surg 2012;94:1802-9. [Crossref] [PubMed]
- Salah S, Watanabe K, Park JS, et al. Repeated resection of colorectal cancer pulmonary oligometastases: Pooled analysis and prognostic assessment. Ann Surg Oncol 2013;20:1955-61. [Crossref] [PubMed]
- Welter S, Jacobs J, Krbek T, et al. Long-Term Survival After Repeated Resection of Pulmonary Metastases From Colorectal Cancer. Ann Thorac Surg 2007;84:203-10. [Crossref] [PubMed]
- Sponholz S, Schirren M, Baldes N, et al. Repeat resection for recurrent pulmonary metastasis of colorectal cancer. Langenbecks Arch Surg 2017;402:77-85. [Crossref] [PubMed]
- Cheung FPY, Alam NZ, Wright GM. The past, present and future of pulmonary metastasectomy: A review article. Ann Thorac Cardiovasc Surg 2019;25:129-41. [Crossref] [PubMed]
- Hirai F, Kinoshita I, Matsubara T, et al. Which primary organ is most suitable for performing pulmonary metastasectomy? Anticancer Res 2018;38:1041-5. [PubMed]
Cite this article as: Forster C, Ojanguren A, Perentes JY, Zellweger M, Migliore M, Krueger T, Abdelnour-Berchtold E, Gonzalez M. Personalized approach for video-assisted thoracic surgery lung metastasectomy. Video-assist Thorac Surg 2020;5:22.


