Uniportal VATS segmentectomy
Introduction
Segmentectomy has been subject to much oncological controversy. Despite this, due to flaws in the original Ginsberg trial, mounting evidence suggests that for the correct patient and indication it can have equivalent oncological benefit to lobectomy. So far, however, this evidence is limited to systematic review and meta-analysis of mainly retrospective data or prospective cohorts (1). Two controlled trials are due to report imminently on 1,106 patients with <2 cm and 697 patients with T1a non-small cell lung cancer randomised to lobectomy versus segmentectomy. Both have already reported perioperative safety outcomes with a longer air leak being the only significantly more common complication of segmentectomy in the Japanese trial (2,3). In their wake a further multi centre trial has begun in the USA in 2015 but the final outcomes for this are projected in 2031 (4). Despite this paucity of quality data, segmentectomy remains a recommendation in international guidelines in selected patients (5-7).
The purported benefits are for patients with poor lung function unable to tolerate lobectomy. The reduction in post-operative lung function is thought to be significantly less, as is the effect on post-operative exercise capacity (8,9). Thus allowing an increased resection rate in a group of patients previously deemed unfit (10).
With the results of the National Lung Screening Trial has come the introduction of lung cancer screening programmes across the world (11). This is projected to lead to an increase in the number of detected lesions which are small in size and carry diagnostic uncertainty. Segmentectomy has been touted as a means of diagnosis and cure in these early stage, screen detected lesions.
The benefit of thoracoscopic surgery has been shown in lobectomy in large propensity matched studies and meta-analyses with shorter hospital length of stay, pain and perioperative morbidity/complications (12-16). This theory of minimally invasive surgery has been extrapolated further into uniportal surgery i.e., minimise the number and size of incisions for further benefit to the patient’s recovery. Whilst this theoretical benefit has not been proven in large studies, multiple suggest it is non inferior in terms of oncological outcome—completeness of resection and lymph node dissection or safety (17).
Uniportal VATS segmentectomy aims to take this further by combining an ultra-minimally invasive approach with parenchymal sparing surgery.
History of uniportal surgery
Minor thoracic surgery procedures have been reported in the literature beginning in 1998 with Yamamoto’s publication of pneumothorax surgery through a single axillary incision (18). As technology progressed, with the increasing uptake of all VATS surgery, so did the ability of surgeons to perform more complex uniportal procedures. This culminated in the first uniportal VATS lobectomy at La Coruna Hospital in June 2010 (19). Since then the complexity of surgery available to the skilled uniportal VATS surgeon has grown to cover almost all the thoracic surgical repertoire and uniportal VATS is practised worldwide. The first reported uniportal VATS segmentectomy was of the S6 segment in combination with the Right Upper lobectomy also in Coruna Hospital, Spain in 2012 (20).
Technology
As technology advances so does the ability of the surgeon to perform more complex procedures safely and with good outcomes. In VATS segmentectomy in particular the most important advances have been in imaging, localisation techniques and instrumentation/operative technology.
With improvements in imaging and software the surgeon now has access to the individualised precise anatomy of the patient from the comfort of the computer chair. Less anatomical “surprises” reduce complications and technical difficulties intraoperatively. 3D reconstruction of CT scans showing the exact segmental anatomy of bronchi and vessels is enabling surgeons to plan more complex procedures. Where previously this was limited by time intensity for the radiologist and expensive, cumbersome software, newer programs allow surgeons to quickly reconstruct images themselves. Aside from assessment of vessel/bronchus anatomy, it is proposed to improve assessment of the lesion distance from the intersegmental planes, thus improving the oncological success of operations (21,22). 3D Printing of the precise patient segmental anatomy, in selected cases, can assist surgeons in physically planning an operation, using materials of a similar consistency to improve intraoperative confidence (23).
Segmentectomies are being touted for the very same small lesions that can be difficult to find during VATS surgery and even open bimanual palpation. Thus the utilisation of localisation markers, mechanical (hookwires, coils) and chemical (methylene blue, indocyanine green, radioactive markers) with the added benefit of a hybrid theatre, has reduced surgical conversion rates and improved theatre efficiency (24).
Techniques to delineate the vascular segmental planes, such as indocyanine green injection, help the surgeon perform a more accurate anatomical resection without the difficulties inflation/deflation of the lung can cause (25).
Indication
- Tumours <2 cm;
- Tumour plus circumferential margin of greater than size of tumour completely contained within anatomic boundaries of segment;
- No N1 disease.
We recommend this in high risk patients (borderline performance status/poor lung function), pure GGOs <2 cm or in patients where metastasectomy cannot be completed as a wedge resection with adequate margins.
Patients will need to be counselled preoperatively for the need of completion lobectomy vs. surveillance in cases where post-operative histology indicates inadequate resection margins, primary lung cancer greater than 3 cm, N1 disease or single station N2 disease.
Position of patient
After induction of general anaesthesia and confirmation of single lung ventilation, preferably via double lumen endotracheal intubation, the patient is turned to the corresponding lateral decubitus position. Some units are now routinely performing uniportal VATS segmentectomies through non intubated anaesthesia with good results and a reduction in anaesthesia related complications (26,27).
Flexion of the table at the level of the xiphoid process, or a soft bolster to achieve the same effect, helps to widen intercostal spaces and move the hips of the patient to allow for easier instrumentation (Figure 1).
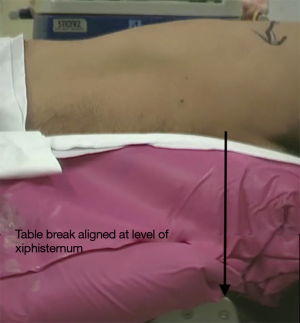
The shoulder should be anteriorly flexed and the arm bent at the elbow so that there is space for the operator. It can be supported with a padded armrest. A combination of soft rolls, beanbag and strapping are used to secure the patient on the table.
Incision
Surface markings of the inferior tip of the scapula, subxiphoid process, anterior border of latissimus dorsi and lateral margin of the breast in women are noted (Figure 2). Intercostal spaces are counted from 2nd down or from costal margin upwards. It is strongly recommended that the positioning is done in a similarly precise manner on every occasion to give the highest chance that the incision’s location is correct.
A 3–4 cm incision should be placed between the anterior and posterior axillary lines as per the Uniportal VATS Interest Group (UVIG) consensus statement (28). The space recommended for each segment, either the fourth or fifth intercostal space, will be described below. During left uniportal VATS a more lateral port position will help instruments avoid the heart during insertion. Placing the port in the same place each time during the learning curve will help develop skills faster.
Position and equipment
The surgeon stands at the front of the patient with the scrub nurse standing at the back. The assistant can be positioned at the front or back—though most commonly this is anterior of the patient (28-30). When working in the lower half of the thorax we recommend the assistant being on the left side of the surgeon and vice versa. The screens should be positioned for surgeon and assistant comfort.
Wound protector use is recommended for easier insertion of instruments, reduced soiling of the camera and to prevent wound contamination (28,30). A 5 or 10 mm 30 degrees scope is recommended and should always be kept in the lateral/uppermost portion of the wound, creating a space below for bimanual instrumentation (29,30). It is essential that the assistant retract the lung with one hand and hold the camera ideally in the dominant hand in a comfortable position.
Energy devices are described in the literature as being used for dissection and division of smaller pulmonary artery and vein branches (<7 mm) (31). In our experience, for safety reasons, we recommend the use of a polymer clip proximally or a tie when the diameter of the vessel is more than 5 mm. For the distal part of the vessels we normally prefer the use of energy devices (a distal clip could interfere with stapler firing during the intersegmental plane division). The authors find that, with practice, once a certain level of expertise is reached, energy devices can help to reduce operating time by increasing the surgeon’s efficiency during dissection.
The benefit of instruments designed specifically for uniportal VATS cannot be understated. At the authors’ institutions the basic instruments used for a standard segmentectomy will be a long curved suction, a distally articulating lung grasper and an energy device being used for the majority of dissection.
Curved tip stapler technology is useful for all components where branches are small and angles awkward.
The table should be moved to assist exposure—e.g., patient tilted posteriorly when exposing anterior mediastinal pleura. Reverse Trendelenberg for the majority of procedures is helpful unless dissecting the inferior pulmonary ligament/vein.
General rules for segmentectomy
The segmental vein, artery and bronchus should be individually dissected, taking care to always include the stumps of these three structures in the segmental resection (29,30).
Before dividing the bronchus, clamp the segmental branch and check insufflation with the anaesthetist to ensure the resection segment remains collapsed and all others inflate.
Common segmentectomies
Below we will cover, in brief, individual segmentectomies and the common combined segmentectomies—this issue covers Right sided segmentectomies in much greater detail in the later sections.
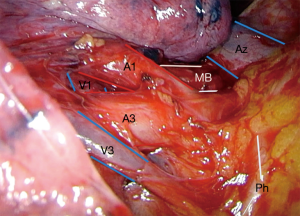
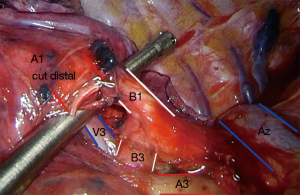
S1 right upper lobe apical segmentectomy (Figures 3 and 4: right, Video 1 shows a left upper lobe apical segmentectomy via uniportal VATS)
- 4th ICS.
- Divide anterior mediastinal pleura.
- Dissect Apical segmental vein by dissecting the Upper lobe vein distally and divide.
- Apical segmental artery next.
- Followed by bronchus.
- Identify segmental margins as above and complete segmental fissures.
S2 right upper lobe posterior segmentectomy
- 4th ICS.
- If fissure is complete—dissect the central vein that runs between the upper (RUL) and the middle lobes (RML). An intersegmental vein runs between S3 and S2 and more distally a vein for S2 that divides into 2 branches. These can be divided separately or together.
- Followed by artery at the confluence of the fissures close to A6.
- The segmental bronchus is found deep and posterior to the divided arterial stump.
- If fissure not complete—dissect bronchus at posterior hilum and identify posterior segmental bronchus and divide.
- Followed by artery.
- To do the vein follow the central vein distally until a parenchymal tunnel is created and this can be opened with staplers to expose the vein all the way to the sub segmental branches.
- Insufflate lung and complete intersegmental fissures.
S3 right upper lobe anterior segmentectomy (Figures 3 and 4)
- 4th ICS.
- Divide anterior mediastinal pleura.
- Dissect anterior segmental vein by dissecting the Upper lobe vein distally and divide.
- Anterior segmental artery next.
- Followed by bronchus.
- Identify segment margins as above and complete segmental fissures.
S6 right lower lobe apical segmentectomy
- 5th ICS, Grasp apical segment and retract caudally.
- If fissure complete dissect and divide apical segmental artery. If fissure partially complete but artery identifiable complete posterior fissure with stapler.
- Followed by bronchus dissection and division immediately posteromedial to artery.
- Apical segmental vein most posteriorly.
- If fissure incomplete dissect bronchus first posteriorly followed by vein or artery in either sequence.
- Identify segment margins as above and complete segmental fissures.
S7-10 basal segmentectomy principles
- If the lesion is not easy to find or palpate we strongly recommend marking the lesion e.g., hookwire or hybrid technology for intraoperative localisation.
- 5th ICS, Expose fissure.
- Dissect out all branches of Lower lobe basilar artery to identify appropriate segmental artery and divide.
- Divide inferior pulmonary ligament and dissect inferior pulmonary vein distally into segmental divisions. Divide appropriate vein.
- Segmental bronchus lies deep to vein branch and can now be divided.
- Identify segment margins as above and complete segmental fissures.
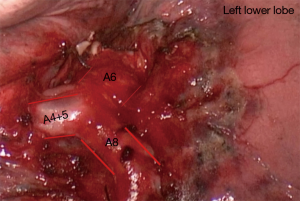
S4-5 Left Lingulectomy (Figure 5)
- Anterior mediastinal pleura divided.
- Lingula vein dissected and divided.
- Lingula artery dissected and divided (artery can be divided first if stapler angles are easier).
- Posterior to vein lingula bronchus can be found, dissected and divided after the usual checks.
- Segmental fissure is completed after insufflation.
S1-3 left apical trisegmentectomy (Video 2)
- Anterior mediastinal pleura divided, upper lobe vein and artery exposed fully.
- Trisegmental vein dissected and divided after confirming lingula vein intact.
- S1+3 artery dissected and divided (artery can be divided first if stapler angles are easier)
- The small S2 posterior artery is divided next.
- Posterior to vein, straddled by the stumps of the A1+3 stump and the vein stump, the trisegment bronchus can be found, dissect here and divide after confirming lingula bronchus intact.
- Segmental fissure is completed.
Conclusion
As the results of the two segmentectomy in Stage 1 lung cancer trials are eagerly awaited, the drive for the ideal surgery that has the lowest perioperative risk, impacts the lives of patients the least functionally and provides the best oncological outcomes continues. Uniportal VATS segmentectomy, from its early inception, has been part of that drive. With continuing technological improvements and a large network of supporting surgeons, its techniques continue to be optimised as it moves to become part of routine lung cancer surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Hitoshi Igai) for the series "Uniportal VATS Segmentectomy" published in Video-Assisted Thoracic Surgery. This article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2020.02.05). The series “Uniportal VATS Segmentectomy” was commissioned by the editorial office without any funding or sponsorship. DGR serves as the unpaid editorial board member of Video-Assisted Thoracic Surgery from Jun 2019 to May 2021. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jsseldijk M, Shoni M, Siegert C, et al. Oncological outcomes of lobar resection, segmentectomy and wedge resection for T1a non-small cell lung carcinoma: a systematic review and meta-analysis. Seminars in Thoracic and Cardiovascular Surgery. Semin Thorac Cardiovasc Surg 2020;32:582-90. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- Flores R, Henschke C, Taioli E, et al. P2.06-045 Initiative for Early Lung Cancer Research on Treatment (IELCART). J Thorac Oncol 2017;12:S1100. [Crossref]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [Crossref] [PubMed]
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65:iii1-iii27. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-e190S.
- Echavarria MF, Cheng AM, Velez-Cubian FO, et al. Comparison of pulmonary function tests and perioperative outcomes after robotic-assisted pulmonary lobectomy vs segmentectomy. Am J Surg 2016;212:1175-82. [Crossref] [PubMed]
- Charloux A, Quoix E. Lung segmentectomy: does it offer a real functional benefit over lobectomy? European Respiratory Review 2017 26: 170079. Am J Surg 2016;212:1175-82.
- Yang CF, D'Amico TA. Thoracoscopic segmentectomy for lung cancer. Ann Thorac Surg 2012;94:668-81. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Paul S, Isaacs AJ, Treasure T, et al. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER-Medicare database. BMJ 2014;349:g5575. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared to thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Yamamoto H, Okada M, Takada M, et al. Video-assisted thoracic surgery through a single skin incision. Arch Surg 1998;133:145-7. [Crossref] [PubMed]
- Gonzalez D, Paradela M, García J, et al. Single-port video assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Mendez L, et al. Single-port video-assisted thoracoscopic anatomic segmentectomy and right upper lobectomy. Eur J Cardiothorac Surg 2012;42:e169-71. [Crossref] [PubMed]
- Chen-Yoshikawa TF, Date H. Update on three-dimensional image reconstruction for preoperative simulation in thoracic surgery. J Thorac Dis 2016;8:S295-S301. [PubMed]
- Yang Q, Xie B, Hu M, et al. Thoracoscopic anatomic pulmonary segmentectomy: a 3-dimensional guided imaging system for lung operations. Interact Cardiovasc Thorac Surg 2016;23:183-9. [Crossref] [PubMed]
- Matsumoto K, Yamasaki N, Tsuchiya T, et al. Three-dimensional (3D) bronchial tree model for bronchial resection with pulmonary segmentectomy. J Thorac Dis 2018;10:E179-82. [Crossref] [PubMed]
- Zaman M, Haris B, Woo C, et al. In patients undergoing video-assisted thoracoscopic surgery excision, what is the best way to locate a subcentimetre solitary pulmonary nodule in order to achieve successful excision? Interact Cardiovasc Thorac Surg 2012;15:266-72. [Crossref] [PubMed]
- Mehta M, Patel Y, Yasufuku K, et al. Near-infrared mapping with indocyanine green is associated with an increase in oncological margin length in minimally invasive segmentectomy. J Thorac Cardiovasc Surg 2019;157:2029-35. [Crossref] [PubMed]
- Wang BY, Liu C, Hsu P, et al. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: a propensity-matched analysis. Ann Surg 2015;261:793-9. [Crossref] [PubMed]
- Liu CY, Hsu PK, Chien HC, et al. Tubeless single-port thoracoscopic sublobar resection: indication and safety. J Thorac Dis 2018;10:3729-37. [Crossref] [PubMed]
- Bertolaccini L, Batirel H, Brunelli A, et al. Uniportal video-assisted thoracic surgery lobectomy: a consensus report from the Uniportal VATS Interest Group (UVIG) of the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;56:628-9. [Crossref] [PubMed]
- Gonzalez-Rivas D. Single incision video-assisted thoracoscopic anatomic segmentectomy. Ann Cardiothorac Surg 2014;3:204-7. [PubMed]
- Hernandez-Arenas LA, Purmessur RD, Gonzalez-Rivas D. Uniportal video-assisted thoracoscopic segmentectomy. J Thorac Dis 2018;10:S1205-14. [Crossref] [PubMed]
- Goudie E, Oliveira RL, Thiffault V, et al. Phase 1 Trial Evaluating Safety of Pulmonary Artery Sealing With Ultrasonic Energy in VATS Lobectomy. Ann Thorac Surg 2018;105:214-20. [Crossref] [PubMed]
Cite this article as: Mahendran K, Hernandez-Arenas LA, Gonzalez-Rivas D. Uniportal VATS segmentectomy. Video-assist Thorac Surg 2020;5:36.