
The ethics of robotic surgical systems is a conversation of informed consent
Introduction
Since its approval for use in surgical procedures in 2000, surgeons’ and patients’ fascination with and desire for innovation have driven robotic techniques to be fully integrated into the armamentarium of thoracic surgery. While robotic surgery will likely never become the required standard of care for the surgical approach to the chest due to restrictions in access, it is a very desirable technique to patients (1). The ethical considerations for use of the robotic system are numerous. In addition to areas typically covered in a surgeon’s informed consent conversation, a few additional topics should be discussed when performing an operation with a robotic system.
Ethics context
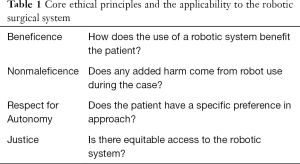
The ethics of a robotic system cannot be discussed without using the ethical principles of beneficence, nonmaleficence, respect for autonomy, and justice (2). Clinical ethics analysis was concisely summarized utilizing the popular Four Boxes model which integrates the ethical principles more comprehensibly (2,3). When looking at these principles in a surgical conversation, beneficence is the determination of whether an intervention is beneficial to a patient. Nonmaleficence is the desire for no harm to come from the surgical intervention. Respect for autonomy permits patients to make their own decisions after being informed of the options. Justice is equitability of access and resources. When discussing the utilization of robotic systems in surgery, the ethical principles, even without clearly being stated, guide preoperative surgical conversations regularly (Table 1). These are routinely discussed preoperatively under the guise of informed consent.
Full table
Informed consent
Informed consent is the process of giving the patients adequate information so they can autonomously give permission for interventions. While surgery with a robotic system requires the same informed consent conversation required for any intervention, a few additional topics need to be covered. It is widely understood that consent is no longer a signature on a piece of paper, but a conversation. The three fundamental criteria that must be present to obtain appropriate informed consent require the patient to have the capacity to make decisions, to be adequately informed, and not to be coerced (4). The issue of whether a patient can ever truly provide informed consent has been previously debated (5,6). Although also debated, it is our current practice that patients should be as informed as a reasonable patient would desire (6-10). Within informed consent, especially with a robotic system, multiple areas need to be discussed to give the patient the appropriate knowledge to be truly informed. Topics that are specific to use of a robotic system include patient misconception, robotic experience, access to robotic systems, and surgeon preference (Figure 1). Areas discussed during any informed consent include beneficence, nonmaleficence, autonomy, and alternatives (Figure 2).
Patient misconception
The concept of a robot involved in an operation is novel, exciting, and desired by many patients. Patients frequently want the least invasive and most modern technology in part of their treatment. In fact, patients drive innovation due to their gravitation toward new and less invasive operations (1). This desire fosters advancement and rapid industry implementation of what is perceived as superior by patients. Historically, the influence of patient preference on innovative procedures was noted with the development and rapid utilization of the laparoscope for cholecystectomy (11). The desire for laparoscopic cholecystectomy was so great that a randomized control trial was initially unable to be performed; patients did not want to be randomized and risk undergoing the more invasive open operation. The robotic system in surgery is currently a robotic-assisted operation; a slave device that is nothing more than a tool and an extension of the surgeon (12). Although the robotic system intermediates between the surgeon and the patient, there is no autonomous function and it is simply an extension of the surgeon. Frequently, patients inappropriately think a robotic system is going to autonomously intervene on their disease. While robotic surgery is no longer new, its proven advantages over video-assisted thoracoscopic surgery are controversial. Although the hype of a robotic technique deserves caution, it does not imply that a robotic system should not be used in thoracic surgery.
Robotic experience
Physician experience is included in the scope of informed consent and it is considered ethically appropriate to disclose (2). There is variability in robotic system experience among thoracic surgeons. As a relatively new technology, there are surgeons starting to use the robotic system without significant experience. Some surgeons are trained to operate robotic-assisted in the same way they are trained to operate video-assisted; it is integrated into the training structure. For most surgeons, however, the development of the robotic platform came after their surgical training and therefore an out-of-training learning curve is experienced. This well-described learning curve is known to exist with robotic systems and every surgeon is variably located on that curve (13). The initial steep learning curve for robotic surgery is thought to be overcome after 15 to 95 cases (13). As surgeon experience develops and one matures out of the learning curve, discussion of the number of cases previously performed is often done at the prompting of the patient. Whether it is early in a surgeon’s learning curve or at the request of the patient, appropriate information regarding robotic experience should be disclosed to permit the patient to make an informed decision (14).
Access to robotic systems
Across institutions, robotic access is variable. At some institutions, with a plethora of robotic systems, or with the prioritization of thoracic surgery use on the robot, access is unrestricted. In other locations with few robotic systems shared across multiple disciplines, the scheduling of an operation may in fact be delayed by having it performed robotically. There can often be a bottleneck at the scheduling of robotic operations due to access. Especially when dealing with cancer patients, the time to operation is of utmost importance (15). These factors should be discussed as they may weigh into a patient’s ultimate decision on facility, surgeon, and approach.
Surgeon preference
It is appropriate if the reason for utilization of a robotic system is simply due to surgeon preference. Nevertheless, this should be disclosed to the patient. As surgeons, we do many things out of preference—the instruments chosen, patient positioning, and brand of devices. The operative approach recommended to a patient relies on surgeon judgment and likelihood of success with a technique. The controversy of using a robotic system with unclear benefit for patients was paralleled when discussed in the setting of laparoscopic cholecystectomy versus open cholecystectomy (16). Although there is some recognized benefit to the utility of the robotic system for tumors of the mediastinum, the conclusions drawn here are similarly applicable to thoracic operations. The robotic system has proven non-inferiority to video-assisted thoracic surgery, but not always added benefit to the patient. It is argued that the robotic system can be used for skill advancement of the surgeon or comfort of the surgeon if added harm is not caused to the patient (16). The robotic platform boasts less muscle fatigue and more ergonomic positioning of the surgeon in a seated position (17). Physicians historically recommend treatment for the benefit of patients and any recommendation for the robotic system, outside of direct patient benefit, needs to be disclosed to the patient (16). Whether it is for surgeon preference, robotic skill development, or comfort of the surgeon, the true reason for robotic recommendation must be discussed.
Beneficence
The primary goal of all indicated medical treatment is to benefit the subject of that treatment. No surgery should ever be done for the sole benefit of a surgeon, health system, or the patient’s family. Similarly, robotic surgery should be performed to benefit the patient. Some of that benefit is simply due to the operation and is not affected by the technique or approach—be it open or minimally invasive. Although controversial, there are some demonstrated benefits of robotic-assisted thoracic surgery over video-assisted thoracic surgery including shorter length of stay, improved lymph node dissection, lower conversion-to-open rate, and lower 30-day complication rate (18,19).
Nonmaleficence
Surgery always carries risks. These risks typically are discussed with the patient preoperatively. But the goal of an operation is never to intend to do harm. As the surgical mantra states, the only way to operate without complications is to not operate. Although complications occur, none are intended or expected. It is the surgeon’s responsibility to ensure nonmaleficence during the implementation of a robotic practice by participating in sufficient training, beginning with appropriate cases, and maintaining a low threshold for conversion to an open or thoracoscopic approach. If surgical risks outweigh the potential benefits, the case is typically not performed. Often for surgery, this risk is upfront, immediate or near-immediate. Alternatives to surgery often have risks over time—like local cancer recurrence after stereotactic ablative radiotherapy in lieu of surgical resection (20,21).
Autonomy
Patient autonomy in medicine has been increasing over the recent decades. The paternalism that previously enshrouded medical consults is on the decline (22). With the improved access to information, often via the internet, patients often come to meet their care teams with a preconceived idea of what treatment they should receive. Rather than the physician being the primary source of the treatment, the physician now often has to re-establish the truth around the patient’s diagnosis prior to making a recommendation. Only after that can the patient make an autonomous, but guided, choice. Additionally, no surgery is performed purely because a patient requests an operation as no surgeon is forced to perform an operation just because a patient may request it. Guiding indications for operations and surgical judgment still supersedes patient autonomy.
Alternatives
If appropriate for a patient’s individual pathology, a surgical conversation of informed consent discusses the specific alternate approaches for an operation. The alternatives to a robotic approach, outside of non-operative options, are open and video-assisted. But at times, the different minimally invasive approaches must be discussed including video-assisted thoracoscopic surgery and robotic-assisted thoracoscopic surgery. There are clear cost differences associated with robotic surgery, video-assisted thoracic surgery, and open surgery (18,23). Cost should be mentioned when discussing alternatives if they are passed on to the patient. Typically, in the United States, the costs of an operation are bundled and billed through the insurance company and will not largely impact the patient directly. It is argued that the upfront cost of the robotic system can be offset by the noted improved post-operative outcomes (23). Intra-institutional cost aside, if one operative approach is going to financially affect a patient more than another, the patient should be made aware of this when discussing alternatives.
Summary
True informed consent, especially in robotic surgery, involves a vast amount of information. Outside of simply providing information, the surgeon needs to provide time for the patient to engage and ask questions as specific patient concerns cannot be fully anticipated (24). The appropriate content and sufficient time provide patients with the proper autonomy to accept the robotic approach or look for an alternative opinion (16).
Conclusions
Patients respect the doctor-patient relationship and a surgeon must be upfront about the role of the robotic system in an operation. Transparency is of utmost importance and being open with patients when planning an operation utilizing the robotic system is equally important. Surgeons with a desire to offer cases utilizing the robotic system need to be honest with themselves and with their patients who seek and undergo robotic surgery. To appropriately provide informed consent for surgery using a robotic system, it is imperative that surgeons include in the conversation common patient robotic misconceptions, the surgeon’s own robotic experience, how access to robotic systems may effect operation timing, and if the robotic system is being offered due to the surgeon’s preference.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Video-Assisted Thoracic Surgery for the series “Robotic Surgery for Lung Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2020.02.02). The series “Robotic Surgery for Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. SCW served as the unpaid Guest Editor of the series. SCW reports non-financial support from Intuitive Surgical, outside the submitted work; EAD reports non-financial support from Intuitive Surgical, outside the submitted work; AWK reports personal fees from Medtronic, non-financial support from Roche Genentech, personal fees and non-financial support from Olympus, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wightman SC, Angelos P. Ethical aspects of a video-assisted thoracoscopic surgery practice. J Vis Surg 2017;3:8. [Crossref] [PubMed]
- Jonsen A, Siegler M, Winslade W. Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine. Seventh Edition. New York: McGraw Hill Professional, 2006.
- Wightman SC, Angelos P. An organized approach to complex ethical cases on a surgical service. World J Surg 2014;38:1664-7. [Crossref] [PubMed]
- Cocanour CS. Informed consent-It's more than a signature on a piece of paper. Am J Surg 2017;214:993-7. [Crossref] [PubMed]
- Boyd K. The impossibility of informed consent? J Med Ethics 2015;41:44-7. [Crossref] [PubMed]
- O'Neill O. Some limits of informed consent. J Med Ethics 2003;29:4-7. [Crossref] [PubMed]
- Spatz ES, Krumholz HM, Moulton BW. Informed Consent and the Reasonable-Patient Standard-Reply. JAMA 2016;316:993-4. [Crossref] [PubMed]
- Drolet BC, Brower JP. Informed Consent and the Reasonable-Patient Standard. JAMA 2016;316:993. [Crossref] [PubMed]
- Main BG, McNair A, Blazeby JM. Informed Consent and the Reasonable-Patient Standard. JAMA 2016;316:992-3. [Crossref] [PubMed]
- Spatz ES, Krumholz HM, Moulton BW. The New Era of Informed Consent: Getting to a Reasonable-Patient Standard Through Shared Decision Making. JAMA 2016;315:2063-4. [Crossref] [PubMed]
- Neugebauer E, Troidl H, Spangenberger W, et al. Conventional versus laparoscopic cholecystectomy and the randomized controlled trial. Cholecystectomy Study Group. Br J Surg 1991;78:150-4. [Crossref] [PubMed]
- Lanfranco AR, Castellanos AE, Desai JP, et al. Robotic surgery: a current perspective. Ann Surg 2004;239:14-21. [Crossref] [PubMed]
- Pernar LIM, Robertson FC, Tavakkoli A, et al. An appraisal of the learning curve in robotic general surgery. Surg Endosc 2017;31:4583-96. [Crossref] [PubMed]
- Ganai S. Disclosure of surgeon experience. World J Surg 2014;38:1622-5. [Crossref] [PubMed]
- Coughlin S, Plourde M, Guidolin K, et al. Is it safe to wait? The effect of surgical wait time on survival in patients with non-small cell lung cancer. Can J Surg 2015;58:414-8. [Crossref] [PubMed]
- Angelos P. Interventions to Improve Informed Consent: Perhaps Surgeons Should Speak Less and Listen More. JAMA Surg 2019; [Epub ahead of print]. [PubMed]
- Rodrigues Armijo P, Huang CK, Carlson T, et al. Ergonomics Analysis for Subjective and Objective Fatigue Between Laparoscopic and Robotic Surgical Skills Practice Among Surgeons. Surg Innov 2020;27:81-7. [Crossref] [PubMed]
- Novellis P, Bottoni E, Voulaz E, et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: comparison of costs and outcomes at a single institute. J Thorac Dis 2018;10:790-8. [Crossref] [PubMed]
- Reddy RM, Gorrepati ML, Oh DS, et al. Robotic-Assisted Versus Thoracoscopic Lobectomy Outcomes From High-Volume Thoracic Surgeons. Ann Thorac Surg 2018;106:902-8. [Crossref] [PubMed]
- Nelson DB, Tayob N, Nguyen QN, et al. Local failure after stereotactic body radiation therapy or wedge resection for colorectal pulmonary metastases. J Thorac Cardiovasc Surg 2019;158:1234-41.e16. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Skowron KB, Angelos P. Surgical Informed Consent Revisited: Time to Revise the Routine? World J Surg 2017;41:1-4. [Crossref] [PubMed]
- Singer E, Kneuertz PJ, D'Souza DM, et al. Understanding the financial cost of robotic lobectomy: calculating the value of innovation? Ann Cardiothorac Surg 2019;8:194-201. [Crossref] [PubMed]
- Angelos P. Can robotic approaches be justified for the benefit of surgeons? Surgery 2017;161:639-40. [Crossref] [PubMed]
Cite this article as: Wightman SC, David EA, Atay SM, Kim AW, Angelos P. The ethics of robotic surgical systems is a conversation of informed consent. Video-assist Thorac Surg 2020;5:24.




