Techniques for robotic lung resection
Introduction
Early stage lung cancer in the appropriate surgical candidate is best treated with an anatomic resection and mediastinal lymph node dissection for staging. Minimally invasive surgical approaches have been steadily replacing open thoracotomy approach over the years. Video assisted thoracoscopic surgery (VATS) has proven to be safe and oncologically equivalent to open surgery (1-3). The benefits of VATS over open thoracotomy for pulmonary resections include less post-operative pain, shorter hospital stays, decreased blood loss, and an increased ability to tolerate adjuvant therapy (4-6). However, despite its effectiveness and proven track record for safety, technical difficulties and limitations—especially in challenging cases—have slowed the adoption of VATS anatomic lung resection. Most recently, robotic assisted surgery (RAS) has gained popularity and acceptance as the alternative minimally invasive surgical approach when performing surgical pulmonary resections (7). Wristed instruments allowing the surgeon full range of motion, instrument stability and precision of motion, and three-dimensional optics are among the numerous technical advantages of RAS over VATS. Patients with morbid obesity, patients who received previous pulmonary resection or redo operations, technically challenging procedures (i.e., segmentectomy), and other atypical cases benefit from minimally invasive operations transformed by these mechanical and optical advantages.
Methods
General considerations
The indications for RAS lung resection are generally the same as those for VATS lung resection. Some may find mediastinal lymphadenectomy and segmentectomy to be facilitated by the robotic approach. Because of the improved optics and range of motion, some surgeons are comfortable using the RAS approach rather than VATS for post-neoadjuvant treatment lung cancer patients. One of the ways in which visualization is enhanced is the use of continuous CO2 insufflation. It is our practice to utilize the AirSeal System with insufflation pressure set to 8mmHg. However, one must be careful in monitoring the patient and communicating with the anesthesiologist, especially when the CO2 insufflation is started. There may be profound hypotension due to tension physiology, especially in dehydrated patients. If this occurs, de-insufflate and bolus the patient with intravenous fluid. Once stable, slowly re-insufflate at lower set insufflation pressure and monitor prior to commencing the operation.
Case setup and positioning
The patient is positioned in lateral decubitus position with the operative side up. A gentle flexion break with slight reverse Trendelenberg position facilitates widening of the intercostal spaces. It is important to note that once robotic arms are in, positioning cannot be changed. A double lumen endotracheal tube should be placed for single lung ventilation with non-ventilated PEEP for the operative lung.
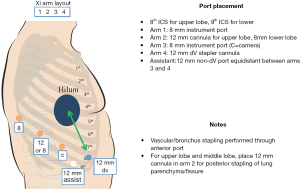
Port placement
Port placement can vary depending on the robot system and preference for three- vs. four-arm technique. In the three-arm technique, there is a camera port and two working ports. In the four-arm technique, there are three working ports. This difference is due to the robotic stapling arm. The robotic stapler has a tendency to rotate around its axis when coming in at a steep angulated direction. Thus, in general, the stapling ports for the DaVinci Xi robotic platform tend to be one or two rib spaces lower compared to the Si system or when using the three-arm technique. In the four-arm technique, three operative port incisions and an assistant port incision are made. For upper and middle lobectomy, the authors prefer to place two 12 mm ports (anterior and posterior) and two 8 mm ports (camera and far posterior port). For lower lobectomy, three 8 mm ports (camera and two posterior) and one 12 mm anterior port are placed (Figure 1).
For upper lobectomy, a 1 cm incision is made through 8th intercostal space at the level of mid-axillary line and a 30-degree scope is inserted. Under direct visualization, additional posterior working ports (12 and 8 mm) are placed 6 cm away along the 8th intercostal space. The surgeon may place working ports one space up or down depending on the patient anatomy and space. The anterior 12 mm port is placed along the 8th space and an assistant port is placed between the camera port and anterior port, just above the diaphragm. For middle lobectomy, the posterior 12 mm working port is placed more posteriorly and one space lower compared to the upper lobectomy. This allows the stapler to come in at a straight angle and facilitates the stapling of the hilar vessels and bronchus.
For lower lobectomy, the ports are placed along the 9th intercostal space, again approximately 6 cm away from each other. Again, posterior ports can be moved one space up or down depending on the patient anatomy and space. The anterior stapling port is placed along the major fissure and the assistant port is placed between the camera port and anterior port just above the diaphragm.
Operative technique
For all lobectomies, the authors recommend taking the inferior pulmonary ligament and opening up the posterior, superior, and anterior mediastinal pleura prior to dissection of the bronchovascular structures as these steps will help identify the anatomy and facilitate the dissection. It is the authors’ approach to use a “fissureless” technique, avoiding fissure dissection to minimize post-operative air leak. If the fissure is complete and well defined, the surgeon may open the fissure if it will help with the operation. During the course of the operation, the dissection is facilitated by removal of anterior and posterior lymph nodes at the bifurcations of vessels and airways before attempting to encircle them. Retraction is performed using two Cadiere graspers, so that the static and dynamic retraction hands may be interchanged easily. The fenestrated bipolar grasper can be used for the majority of dissection. A rolled raytec “cigar roll” is used to aid in hemostasis and blunt sweeping dissection when necessary. A fenestrated tip up is used to pass around bronchovascular structures, and they are encircled with a vessel loop to facilitate passage of the stapling device. The bedside assistant’s role is generally to provide static retraction, suction fluid or blood to provide a clear field, and remove specimens.
Results
Right upper lobectomy
The operation begins by retracting the lower lobe cranially. Release the inferior pulmonary ligament and harvest level 8/9 lymph nodes. Once the lung is retracted anteriorly, dissect the subcarinal space and harvest level 7 lymph nodes. The posterior mediastinal pleural is opened superiorly and the right upper lobe bronchus takeoff and the bronchus intermedius are identified (Figure 2A). The level 11 “sump” lymph node is removed to help identify the anatomy and facilitate encircling the bronchus later. The posterior ascending pulmonary artery (PA) branching to the upper lobe resides just underneath this node so one should use caution when dissecting the node. Surgeons who prefer the “posterior to anterior” approach would encircle and divide the bronchus and dissect anteriorly at this point.
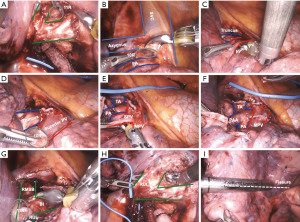
The lung is now retracted caudally and the superior hilar pleura is incised. This allows the right upper lobe bronchus and truncus anterior branch to be exposed. The level 10 lymph node packet between the bronchus and truncus anterior is carefully removed (Figure 2B). This maneuver frees up the tissue behind the truncus branch and helps with encircling the vessel later. The lung is now retracted posteriorly, opening the anterior mediastinal pleura (Figure 2C). A level 10 lymph node just anterior to the anterior truncus branch is removed, as it helps with encircling the truncus anterior branch (Figure 2D).
The key step in the upper lobectomy is encircling and dividing the truncus branch. The fenestrated tip up grasper is blunt and its curve helps carefully negotiate behind the vessel and isolate the artery. The angle of the stapler coming from the anterior port should be as straight as possible, so as not to torque on the vessel. Once the truncus branch is divided, the superior pulmonary vein is isolated (Figure 2E), making sure to identify the middle lobe vein. The vein is divided and the posterior ascending pulmonary artery branch is isolated anteriorly and divided (Figure 2F). The lung is retracted anteriorly and the bronchus is encircled and divided (Figure 2G,H). The posterior and minor fissure is then completed using the stapler (Figure 2I). The superior mediastinum is opened and the 4R nodal packet is removed.
Right middle lobectomy
The inferior pulmonary ligament, mediastinal pleura, and nodes are dissected as mentioned before. The lung is retracted posteriorly exposing the anterior hilum. The anterior mediastinal pleura is opened and the middle lobe pulmonary vein is isolated and divided using the stapler from the posterior port (Figure 3A,B). Just deep to the divided vein, the middle lobe bronchus is identified. Remove the nodes anterior and posterior to the bronchus and dissect around the bronchus (Figure 3C). One must be mindful that the pulmonary artery is deep to the bronchus so care should be taken when getting around the bronchus (Figure 3D). Place a vessel loop around the bronchus and pull up, placing the stapler as proximally as possible to have a short divided middle lobe stump. Once the bronchus is divided, the main body of the PA and middle lobe PA branches are exposed (Figure 3E). It may be easy to dissect around the PA by opening up the fissure around the PA. There are usually one or two large branches of the middle lobe PA. Remove the nodes anterior and posterior to the PA and encircle it using a vessel loop. The stapler is placed from either posterior port or anterior port depending on the angle and the middle lobe PA is divided. The minor fissure and lung parenchyma is divided (Figure 3F,G,H).
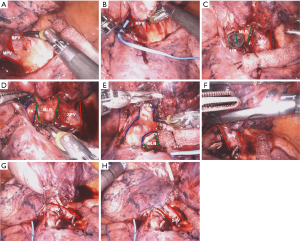
Right lower lobectomy
The inferior pulmonary ligament and the posterior mediastinal pleura are opened. The lung is retracted anteriorly and the lymph node between the inferior pulmonary vein and the bronchus is removed, and posterior space behind the vein is created (Figure 4A). The posterior dissection is continued and the level 11 node at the bifurcation of the upper lobe bronchus and the bronchus intermedius is identified and carefully removed (Figure 4B). The lung is then retracted cranially and posteriorly, the anterior mediastinal pleura is opened up and the node next to the vein is removed, thus creating a path to go around the inferior vein (Figure 4C). Using a fenestrated tip up, encircle the vein using a vessel loop and divide it using a stapler from the anterior port. With the vein divided, the node between the PA and the bronchus is then removed, creating a space behind the bronchus (Figure 4D). The plane between the bronchus and the PA is created. Using a fenestrated tip up, hug around the lower lobe bronchus and encircle the bronchus using a vessel loop, identifying and preserving the middle lobe bronchus (Figure 4E). Place the stapler through the anterior port and divide the bronchus. The lung is retracted superiorly once again. The basilar PA and superior segment PA now visible (Figure 4F). Gently dissect around each vessel and divide using the stapler, being sure to identify and preserve the middle lobe artery (Figure 4G). Complete the fissure using the stapler (Figure 4H,I).
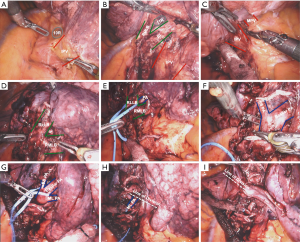
Left upper lobectomy
After releasing the inferior pulmonary ligament and harvesting level 8/9 lymph nodes, the lung is retracted anteriorly and the posterior mediastinal pleural is opened superiorly. The level 11 lymph node that overlies the PA is removed (Figure 5A), and the posterior branch or branches to the upper lobe is/are exposed (Figure 5B). These are encircled and divided. With the posterior PA branches divided, some work on the truncus branch can be done from the posterior exposure (Figure 5C). The lung is then retracted posteriorly and the anterior mediastinal pleura opened. The superior pulmonary vein is isolated and divided (Figure 5D). The level 10 lymph node between the superior pulmonary vein and PA is carefully removed, allowing the truncus branch to be fully exposed, encircled, and divided (Figure 5E). The left upper lobe bronchus is then isolated and divided (Figure 5F). Division of the upper lobe bronchus exposes the remaining anterior facing lingular PA branches (Figure 5G) which are isolated and divided. The fissure is completed using the stapler (Figure 5H). The specimen is removed. Lastly, the level 5 lymph nodes are harvested (Figure 5I), and the subcarinal level 7 lymph node packet is removed.
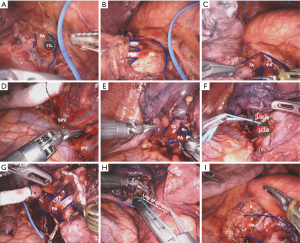
Left lower lobectomy
The inferior pulmonary ligament and the posterior mediastinal pleura are opened as usual (Figure 6A). The lymph node between the inferior pulmonary vein and the bronchus is removed, and posterior space behind the vein is created (Figure 6B). The anterior mediastinal pleura is opened up and the node next to the vein is removed, thus creating a path to go around the inferior vein. Using a fenestrated tip up, gently get around the vein, encircle it using a vessel loop and divide it using a stapler from the anterior port (Figure 6C). The level 10 nodes on top of the PA are removed and avascular plane is developed on the main PA and dissected distally (Figure 6D). The node between the PA and the bronchus is removed, creating a space behind the bronchus (Figure 6E). This maneuver is important for preventing injury while getting around the PA later. The lung is now retracted superiorly and the bifurcation between the lower lobe bronchus and upper lobe bronchus is identified. There is a level 11 node at the bifurcation, and this node is carefully removed, being mindful that the basilar PA is deep to the node (Figure 6F). The plane between the bronchus and the PA is created. Using a fenestrated tip up, hug around the lower lobe bronchus and encircle the bronchus using a vessel loop. Place the stapler through the anterior port and divide the bronchus. The lung is retracted superiorly once again. The basilar PA and superior segment PA are on full display (Figure 6G,H). Gently dissect around each vessel and divide using the stapler. Complete the fissure using the stapler (Figure 6I).
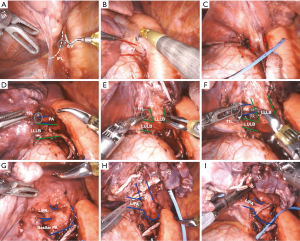
Segmentectomy
The decision between segmentectomy and lobectomy for lung cancer depends on several factors, including size of the lesion, location, presence or absence of PET avid regional N1 nodes, and the patient’s underlying fitness and lung function. The oncologic outcomes comparing the two techniques are being actively investigated (8). The anatomical segmentectomy may be a viable option in patients with a small peripheral lung cancer <2 cm, elderly patients, multi-focal lung lesions, or those with limited lung function.
Left superior segmentectomy
Release the inferior pulmonary ligament and open up the posterior mediastinal pleura as described before. The inferior pulmonary vein is identified and avascular plane is created on top of it (Figure 7A). The dissection is carried distally until the bifurcation to the superior segment is identified (Figure 7B). The vein is then isolated and divided using a stapler. The lung is retracted anteriorly, and the upper lobe bronchus and bronchus intermedius are identified (for the right side). The “sump node” is removed and the plane is created between the bronchus and PA. The lower lobe bronchus is identified and dissection is carried distally until the superior segmental bronchus is identified (Figure 7C). In this figure, clips and ties were used to divide small bridging PA and vein branches. The bronchus is then divided. The superior segment PA is just underneath the bronchus. The exposure can be maximized by opening up the fissure around the vessels. Once fully exposed and isolated, the PA is divided (Figure 7D). Using 10 mg IV ICG, the segmental plane can be delineated (Figure 7E,F). Using the stapler, divide the lung parenchyma, making sure to retract the divided bronchus up and placing the stapler just underneath.

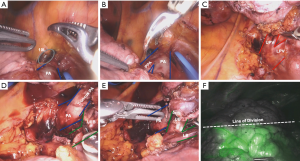
Left upper trisegmentectomy
The inferior ligament and the posterior mediastinal pleura are opened as before. The level 10 node in front of the main PA is dissected and removed (Figure 8A). This allows the avascular plane on top of the main PA to be developed. The lung is retracted down and the superior hilar pleura is incised, opening up the rest of the PA. The dissection is carried on the top of the main PA until the posterior PA branch to the upper segment is identified (Figure 8B). Carefully, the node between the main PA and the PA branch is removed. This creates a plane to get around the PA branch. The stapler is then delivered through the posterior port and the PA branch is divided. The lung is now retracted posteriorly, exposing the anterior hilum. The superior pulmonary vein is identified. The lingular vein is spared and the upper segment vein is isolated and divided (Figure 8C). Once the vein is divided, retract the lung posteriorly further. The truncus branches to upper tri-segment are identified (Figure 8D). Extreme care must be made when getting around this branch as too much traction can lead to avulsion of the PA branch. Once isolated, divide the PA branch. There may be additional small PA branches to the upper lobe segment and they can be divided individually. Identify the upper segment bronchus and lingular bronchus bifurcation (Figure 8E). Encircle and divide the upper segment bronchus. 10mg IV ICG is given and the segmental plane is identified (Figure 8F). Divide the lung parenchyma along the segmental plane.
Discussion
The techniques and approaches to robotic pulmonary resection have evolved as the robotic technology has improved. The first robotic lobectomy described by Melfi et al. in 2002 (9) was performed using a DaVinci S system using the three-arm technique and a “service entrance” incision. The placement of the trocars had to be precise and far away to avoid collision between the arms, and the instruments were rudimentary to perform highly sophisticated and complex operations. There had been a tremendous amount of progress and advancement in the technology since that time to make the operation safer and easier to perform.
Unlike the S and Si system, the Xi system allows docking from the side so that the anesthesiologist had access to the airway more easily. The changes in the docking mechanism and cannulas have made the docking process simpler and more forgiving. In addition, the instruments have improved to make the operation easier and safer. Above all, the fourth arm of the current Xi system allows the surgeon to have full control over the vascular stapling which had been one of the major impediments to the adoption of robotic pulmonary surgery by thoracic surgeons. The advent of the robotic vascular stapler and the fourth arm of the Xi system have moved many surgeons to adopt the four-arm technique and place their ports lower to accommodate for the angle of the stapler.
Interestingly, while the four-arm technique using the Xi system would seem to be the more advantageous, both the three- and four-arm techniques have proponents. The argument for the three-arm technique is that it costs less to use three arms and perform the stapling by the bedside assistant. However, one must have a very experienced and competent bedside assistant to make this technique effective. The four-arm technique allows the surgeon to have full control over all aspects of the operation including vasculature stapling, but financially it is more expensive. There are no comparative data to show whether the three- or four-arm technique is superior. Therefore, the operative approach should be determined by the surgeon’s preference and the experience and support staff involved in the cases.
Conclusions
Robotic anatomical pulmonary resection is a safe, oncologically effective alternative to the video-assisted thoracoscopic approach. The mechanical and optical advantages afforded by the robotic approach may make certain challenging patient populations and technically demanding operations easier and safer to perform.
Acknowledgments
The authors would like to thank Ms. Elena Susan for her assistance with manuscript preparation.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Natalie S. Lui and Sean C. Wightman) for the series “Robotic Surgery for Lung Cancer” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2019.12.06). The series “Robotic Surgery for Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics approval was not required for this study. Individual consent for this study was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Seder CW, Hanna K, Lucia V, et al. The safe transition from open to thoracoscopic lobectomy: a 5-year experience. Ann Thorac Surg 2009;88:216-25; discussion 25-6. [Crossref] [PubMed]
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951-60; discussion 60-1. [Crossref] [PubMed]
- Berry MF, D'Amico TA, Onaitis MW, et al. Thoracoscopic approach to lobectomy for lung cancer does not compromise oncologic efficacy. Ann Thorac Surg 2014;98:197-202. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Nicastri DG, Wisnivesky JP, Litle VR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg 2008;135:642-7. [Crossref] [PubMed]
- Martin JT, Durbin EB, Chen L, et al. Nodal Upstaging During Lung Cancer Resection Is Associated With Surgical Approach. Ann Thorac Surg 2016;101:238-44; discussion 44-5. [Crossref] [PubMed]
- Louie BE, Wilson JL, Kim S, et al. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using The Society of Thoracic Surgeons Database. Ann Thorac Surg 2016;102:917-24. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
Cite this article as: Kim S, Chiu S. Techniques for robotic lung resection. Video-assist Thorac Surg 2020;5:3.