Does low-dose computed tomography screening improve lung cancer-related outcomes?—a systematic review
Introduction
Lung cancer is the second commonest cancer and leading cause of cancer-related deaths in Hong Kong, killing 3,780 patients in 2016 (1). The high mortality rate of lung cancer can be attributed to its generally late presentation, with around 69% of patients presenting at stage III or IV disease and therefore not amenable to surgical treatment (2). It has been postulated that screening can improve lung cancer survival by detection of tumours while they are still in resectable early stages. However, over the years, methods such as sputum cytology and chest X-ray (CXR) have been shown to be inadequately sensitive for screening purposes, while conventional computed tomography (CT) confers an unacceptably high radiation dose (3).
The advent of low-dose CT (LDCT) technology has made it possible to obtain high-quality images with a low radiation exposure (4), and recent studies have investigated the use of LDCT thorax scans to screen for lung cancer with some encouraging results. Based on multiple early trials conducted in the west, the United States and Canada have published guidelines on LDCT lung cancer screening programs for high-risk populations a number of years ago (5,6).
One of the upshots of lung cancer screening is that potentially smaller lesions may be detected and be amenable to the latest forms of minimally invasive thoracic surgery (7). Video-assisted thoracic surgery (VATS) has evolved in recent years, and can now be performed via Uniportal approach, promising less surgical access trauma and better patient recovery than ever before (8,9). In addition, sublobar resection has now been demonstrated to offer equally effective therapy for smaller lung cancers, such as those typically identified by screening (7,10). The advent of LDCT screening appears to have arrived at the optimal time to take advantage of these latest thoracic surgical advances.
As more randomised-controlled trials are emerging in recent years from different countries, including some in Asia, an updated literature review is now due in order to update and refine screening practices (11). The primary objective of this systematic review was to provide an updated assessment of how LDCT screening may impact lung cancer-related outcomes. The secondary objective was to explore the relevance of the existing literature to populations in Asia, where VATS and sublobar resections are especially commonly practiced (12).
Methodology
Search method
Between November and December 2018, literature searches were conducted through the Ovid search engine in the PubMed and the MEDLINE database for original clinical studies, using the MeSH terms (Tomography, X-Ray Computed) AND (lung or pulmonary or non-small cell) AND (cancer or ca or neoplasm or malignancy or tumour) AND (screen or early detection or early diagnosis). The inclusion criteria included retrospective or prospective, and observational or randomised-controlled trials (with an alternative screening protocol or standard care as control) of any sample size, which utilised LDCT thorax scans to screen for primary, non-small cell lung cancer in asymptomatic individuals and published data in English on any combination of outcomes including lung cancer detection rate, invasive intervention rate, false positive rate of CT scans and invasive interventions, staging, and resection rate of diagnosed cancers, and mortality rate. The exclusion criteria included articles that were not published in English or Science Citation Index (SCI) peer-reviewed journals. Titles and abstracts were assessed for relevance to the primary objective, and the resulting full papers were read in their entirety for adherence to the inclusion criteria.
Data extraction and outcome measures
Each study’s authors, publication year and methods were extracted. The outcome measures examined were the lung cancer detection rate, LDCT false positive rate, rate of unnecessary invasive procedures, staging distribution of LDCT-detected lung cancers, resection rate of LDCT-detected lung cancers, and lung cancer-related mortality.
Lung cancer detection rate was defined as the percentage of screened cases detected to have lung cancer. LDCT false positive rate was defined as the percentage of LDCT scan-positive subjects referred for further follow-up, including specialist appointments, repeat scan or more invasive procedures, who ultimately had benign lesions. The rate of unnecessary invasive procedures was defined as the percentage of screened subjects who received invasive procedures, including bronchoscopy, tissue biopsy or surgical resection, who ultimately had benign lesions. Staging distribution of LDCT-detected lung cancers was defined according to the tumour, node, and metastasis classification of lung cancer in the sixth edition of the Cancer Staging Manual by the American Joint Committee on Cancer. Lung cancer-related mortality was defined as the percentage of lung cancer patients who ultimately died from lung cancer-related mortality.
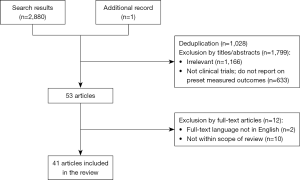
For trials with multiple published articles, the most updated data was used. Figure 1 displays the search methodology.
Results
The initial literature search yielded 2,880 articles, 1,028 of which were duplications. Irrelevant studies, non-clinical trials and reports with no measured outcomes were excluded based on the studies’ titles and abstracts, resulting in 53 articles which were read in their entirety. After excluding articles that were not in English or did not report on this review’s outcome measures, 40 articles reporting (2,13-36) on 27 studies (37-51), and one additional press release with the most updated results of one of the largest trials to date (52), were included in this review. Among the reviewed studies, 17 were observational studies (15 prospective and 2 retrospective) (13-31,53), and 11 were randomised-controlled trials (RCTs) (32-52). Among the RCTs, 10 had published comparative data, three of which compared LDCT screening with CXR screening (32-35) and seven of which compared LDCT screening with no screening (36-45,51,52).
Study designs and baseline characteristics
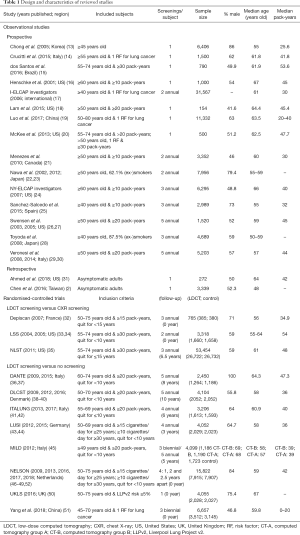
The study design and baseline characteristics of each study are presented in Table 1. In general, the observational studies included both male and female subjects older than 40 years old (median ages 50–67 years old) with at least 10 pack-years (median pack-years 20–53.6) of cigarette smoking history, had screening frequencies ranging from a one-off screening to five annual screenings, and recruited sample sizes of 154 to 3,167 subjects (2,13-31). Most of the RCTs included more males than females subjects, 50- to 75-year-old (median ages 55–67 years old) with at least 20 pack-years (median pack-years 10–54), had screening frequencies ranging from 1 to 5 annual screens, and recruited sample sizes of 654 to 53,454 subjects (32-51). The two largest RCTs (NLST and NELSON) have a combined sample size of more than twice the combined sample size of the rest of the RCTs (35,47).
Full table
Lung cancer detection rate
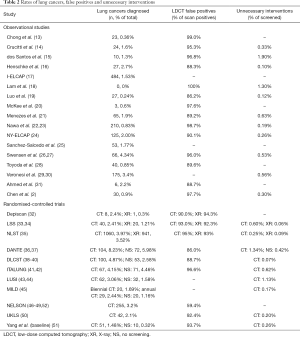
Studies that performed one, two, three, four and five LDCT screenings detected lung cancer in 0–2.7%, 0.8–2.4%, 0.9–4.0%, 3.1–4.2% and 2.4–8.2% of their participants respectively. CXR detected lung cancer in 0.3–3.5% of their participants as reported by 3 RCTs, and 0.3–6.0% of individuals who received no screening were eventually diagnosed with lung cancer as reported by 6 RCTs.
The lung cancer detection rate between LDCT versus CXR screening was compared in one RCT, which showed a significantly higher lung cancer detection rate by LDCT screening (RR 1.03–1.23). The lung cancer detection rate in the LDCT screening versus no screening groups was compared in four RCTs, three of which showed a significantly higher lung cancer detection rate by LDCT (P≤0.001 to 0.042) while one showed no significant difference (RR 95% CI, 0.7–1.3) (Table 2).
Full table
Rates of false positive (FP) and unnecessary invasive procedures
The FP rates were reported to be 59.4–100% for LDCT according to 14 observational studies and nine RCTs, and 92.3–94.3% for CXR according to three RCTs (Table 2).
The percentage of total screened subjects who ultimately received unnecessary invasive procedures for benign lesions was 0.07–1.9% for LDCT according to 11 observational studies and nine RCTs, 0.06–0.09% for CXR according to two RCTs, and 0.42% for no screening according to one RCT (Table 2).
Stages of diagnosed lung cancers
LDCT-detected lung cancers presented 33.3–96.1% at stage I, 0–50.0% at stage II, 0–50.0% at stage III, and 0–36% at stage IV, as reported by 14 observational studies and all 11 RCTs. CXR-detected lung cancers presented 30.7–100.0% at stage I, 0–7.9% at stage II, 0–25% at stage III, and 0–35.6% at stage IV, as reported by three RCTs. Lung cancers diagnosed in subjects receiving no screening presented 9.4–22.2% at stage I, 3.8–30.0% at stage II, 10.0–17.0% at stage III, and 40.0–60.4% at stage IV, as reported by five RCTs (Table 3). Figure 2 displays the staging proportions from RCTs with comparative data.
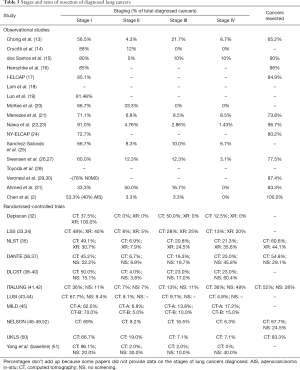
Full table
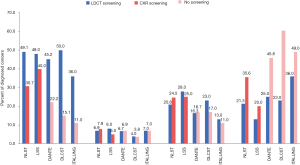
The staging distribution of lung cancers detected by the LDCT versus CXR screening was compared in one RCT, which showed no significant difference (P=0.8). The staging distribution of lung cancers diagnosed in the LDCT screening versus no screening groups was compared in four RCTs, of which three showed a significantly higher proportion of stage I disease detected in the LDCT group (P<0.0001 to <0.001), one showed no significant difference in proportion of stage IV disease (P=0.28), and one showed no significant difference in proportion of advanced disease (Union for International Cancer Control’s stage II or above; P=0.25) (Table 3).
Resection rates of diagnosed lung cancers
The proportion of lung cancers that was resected was 52–100% for LDCT-detected subjects as reported by 11 observational studies and four RCTs, 44.1% for CXR-detected subjects as reported by one RCT, and 28–29.1% for subjects who received no screening as reported by two RCTs (Table 3). The lung cancer resection rate in the LDCT screening versus no screening groups was compared in three RCTs, all of which showed a significantly higher lung cancer resection rate in the LDCT group (P≤0.003).
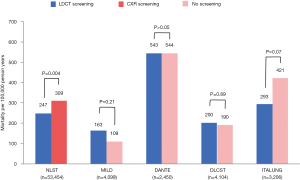
Lung cancer-related mortality
Lung cancer-related mortality in LDCT versus CXR screening groups was compared in one RCT, which showed a significant 20.0% reduction in mortality by LDCT screening compared to CXR screening (P=0.004). Lung cancer-related mortality in the LDCT versus no screening groups was compared in five RCTs, of which one showed a significant 26% reduction in mortality by LDCT screening (95% CI, 9–41%, dataset not yet available and not shown in Figure 3) and four showed no significant difference (P>0.05) (Figure 3).
Discussion
This review of current literature shows that LDCT screening is successful in detecting a high number of lung cancers among a high-risk population, especially when compared to CXR or no screening. While all studies found a high LDCT false positive rate of at least 59.4%, most of these false positive scans did not necessitate any extra procedures, resulting in a low 0.07–1.9% rate of unnecessary interventions for benign lesions. The lung cancers detected by LDCT screening tend to be in their earlier stages, and the proportion of stage I cancers among LDCT-screened subjects is found by most RCTs to be higher than among non-screened subjects. The proportion of LDCT-detected lung cancers amenable to treatment by resection is above 50% for all studies, which is significantly higher than cancers diagnosed in individuals with no screening. The lung cancer-related mortality may be significantly decreased by LDCT screening when compared to CXR or no screening, but this effect was only seen in two of the six RCTs with comparative data (Figure 3).
LDCT has the ability to identify pulmonary lesions as small as 5 mm or below, well before the lesions can cause any clinical symptoms (54). It is therefore not surprising that LDCT leads to a higher detection rate of lung cancers, particularly of earlier-stage lung cancers, as concluded by this review. Lung cancers diagnosed at earlier stages are associated with a higher survival compared to those diagnosed at later stages—in the United Kingdom, the one-year survival rate of stage I cancers has been estimated to be 72.5%, compared to 15.9% for stage IV cancers (55). Survival after VATS for lung cancer in Asia has been reported to be even higher (10,12,56). This increase in survival with earlier-stage cancers is expected because localized cancers can be effectively manged with localized treatment options with curative intent such as VATS and sublobar resection. Our finding that LDCT-detected cancers, compared to non-screened subjects, have a significantly higher proportion of earlier-stage cancers corresponds with the finding that LDCT-detected lung cancers also have a significantly higher resection rate, since surgery remains the treatment of choice for cancers and candidates fit for resection (57). Moreover, identification of earlier, smaller lesions usually means that screening-detected patients are more likely to be candidates for VATS and sublobar resection (7,11).
However, despite facilitating earlier diagnoses and more surgical resections of lung cancers, LDCT screening was only shown by two studies to lead to significantly decreased lung cancer-related mortality. There are two potential reasons to explain this result. Firstly, it is possible that a portion of screen-detected lung cancers might never be clinically significant or cause symptoms before the subject succumbs to other comorbid conditions, suggesting that a high proportion of LDCT-detected cancers in fact represents overdiagnoses. The NLST investigators have attempted to quantify the overdiagnosis rate of LDCT-detected lung cancers, and concluded that the probabilities that any LDCT-detected lung cancer, non-small cell lung cancer and bronchioalveolar lung cancer being an overdiagnosis are 18.5%, 22.5% and 78.9% respectively (58). The extent to which overdiagnosis contributes to the lack of significant decrease in lung cancer-related mortality remains to be determined, but a study estimates that the five-year overall survival for untreated stage I non-small cell lung cancer is only about 6% with a median survival of 9 months, indicating that most early stage lung cancers do require treatment to prolong survival (59). More research defining the radiological or histological features of comparatively indolent cancers and more conservative management of certain subsets of suspicious nodules, could help decrease the rate of unnecessary treatment for otherwise insignificant cancers.
A second reason to explain why not all studies showed a decrease in lung cancer-related mortality by LDCT screening is that the only two RCTs showing a significant mortality decrease are also the ones with the largest sample sizes, with a combined sample size (n=69,276) almost five times that of all the other RCTs (n=13,859). Studies with larger sample sizes, or meta-analyses of data from existing trials, are likely needed to achieve sufficient power to detect the mortality benefit, if present, of LDCT screening. It is therefore likely that LDCT screening does improve lung cancer-related outcomes in some high-risk populations, despite smaller studies not concluding as such.
The rate of unnecessary invasive interventions in LDCT-screened subjects concluded by this review is low, at 0.07–1.9% (with most studies estimating the rate to be below 0.63%; Table 2). This rate of unnecessary interventions is comparable to other, more established screening programs such as mammography screening, which has been estimated to lead to unnecessary breast lesion biopsies in less than 0.66% of screened subjects (60). With this low rate of unnecessary invasive procedures and likelihood that LDCT screening improves lung cancer-related outcomes, multiple countries such as the United States, Canada and the United Kingdom are implementing LDCT lung cancer screening in their healthcare systems.
How applicable is the data from this review to populations outside the West? Both of the largest trials (NLST and NELSON) focused on smokers as the high-risk population for developing lung cancer, but data from these western studies may not be completely applicable to Asian patients, for example. Six of the newer trials included in this review were conducted in Asia, with a generally lower lung cancer diagnostic rate compared to western studies, suggesting that the appropriate target screening population has not yet been delineated among Asians. Focusing on patients of Chinese ethnicity, one of the major differences between lung cancer patients in Hong Kong and the west is that the proportion of non-smokers is higher in the former than the latter (30% vs. 10–15%) (61,62). Indeed, one of the reviewed Chinese studies found a borderline significantly higher lung cancer incidence rate among non-smokers compared to smokers, suggesting that non-smokers should also be included in screening programs of our population (19). Emerging studies are beginning to demonstrate possible biological differences between lung cancers in Asian and Western populations (63,64). More research should be conducted to delineate other significant risk factors for lung cancer in our locale, such as EGFR mutations, to define a more specific target screening population.
Even if LDCT screening led to improved lung cancer-related outcomes with minimal risks, the cost-effectiveness of such a program in our society must also be considered. A widely accepted calculation to assess an intervention’s cost-effectiveness is the incremental cost-effectiveness ratio (ICER), which provides information on the net cost to achieve a unit of health, usually presented as life-years (LY) or quality-adjusted life-years (QALY) gained (65). There is no standardisation of ICER thresholds to inform decision making in Asia. In the United States, an arbitrary upper threshold of USD 50,000/QALY gained has traditionally been used to decide that an intervention is cost-effective (65), but health economists have recently proposed a higher cut-off of USD 100,000/QALY gained (66). In the United Kingdom, the National Institute for Health and Care Excellence (NICE) guidelines recommend an upper threshold of GBP 30,000 (USD 37,948)/QALY gained (67). According to a systematic review of cost-effectiveness studies of LDCT screening, the ICER for screening has been estimated to be USD 1,464 to 2,322,700 (68). Out of the nine reviewed studies, ICER calculated from seven studies is lower than USD 100,000/QALY gained and from five studies is lower than GBP 30,000/QALY gained, suggesting that LDCT screening could be cost-effective in most contexts. Using Hong Kong as an example, because the cost of a LDCT scan in Hong Kong (about USD 255) is lower than the cost reported and used for some western analyses (USD 1,130), the ICER for screening in Hong Kong could potentially be even lower than the currently available numbers, especially if the target screening population is more appropriately defined as suggested above. More locally relevant cost-effectiveness analyses should be conducted to define the health benefits of a LDCT lung cancer screening program in Asia.
Limitations and future directions
A major limitation of this literature review is the lack of consistency in the design of the individual studies included. Heterogeneity between the reviewed studies exists in terms of the screening population selection, screening intervals and study duration. For this reason, we have deliberately avoided performing a meta-analysis combining the studies’ data. Less than half of the trials in the literature review are RCTs, which makes comparing LDCT screening with other forms of screening difficult. Finally, a full review of all the side effects of screening, such as increased radiation and psychological stress of LDCT false positives on the patient, is important to note before a screening program can be suggested, but was not done in this review.
Therefore, future research directions should include standardised and larger-scale randomised-controlled trials conducted in Asian populations, with a particular focus to delineate Asian-specific risk factors for lung cancer in order to properly target high-risk groups in our population. In addition, further studies to more appropriately define LDCT scan-positive lesions and determine scan features more specific to lung cancer, such as high lesion doubling time, can decrease the rate of unnecessary interventions.
Conclusions
LDCT screening is effective in diagnosing early-stage, resectable lung cancers, especially when compared to CXR screening or no screening. LDCT screening may also decrease lung cancer-related mortality with an acceptable rate of unnecessary interventions. Implementation of LDCT lung cancer screening in Chinese and other Asian populations can be considered after more locally relevant screening trials and cost-effectiveness analyses.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Hitoshi Igai) for the series “Uniportal VATS Segmentectomy” published in Video-Assisted Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2020.01.02). The series “Uniportal VATS Segmentectomy” was commissioned by the editorial office without any funding or sponsorship. Dr. Sihoe reports personal fees and non-financial support from Medela AG, Medtronic and Johnson & Johnson, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Authority H. Hong Kong Cancer Registry. 2018. Accessed 28 November, 2018.
- Cheng TY, Cramb SM, Baade PD, et al. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor Characteristics. J Thorac Oncol 2016;11:1653-71. [Crossref] [PubMed]
- Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011;306:1865-73. [Crossref] [PubMed]
- Albert JM. Radiation risk from CT: implications for cancer screening. AJR Am J Roentgenol 2013;201:W81-7. [Crossref] [PubMed]
- Moyer VA, Force USPST. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. [PubMed]
- . Canadian Task Force on Preventive Health C. Recommendations on screening for lung cancer. CMAJ 2016;188:425-32. [Crossref]
- Sihoe AD, Van Schil P. Non-small cell lung cancer: when to offer sublobar resection. Lung Cancer 2014;86:115-20. [Crossref] [PubMed]
- Sihoe AD. The evolution of minimally invasive thoracic surgery: implications for the practice of uniportal thoracoscopic surgery. J Thorac Dis 2014;6:S604-17. [PubMed]
- Sihoe ADL. Uniportal Lung Cancer Surgery: State of the Evidence. Ann Thorac Surg 2019;107:962-72. [Crossref] [PubMed]
- Ji C, Xiang Y, Pagliarulo V, et al. A multi-center retrospective study of single-port versus multi-port video-assisted thoracoscopic lobectomy and anatomic segmentectomy. J Thorac Dis 2017;9:3711-8. [Crossref] [PubMed]
- Sihoe ADL, Cardillo G. Solitary pulmonary ground-glass opacity: is it time for new surgical guidelines? Eur J Cardiothorac Surg 2017;52:848-51. [Crossref] [PubMed]
- Sihoe ADL, Han B, Yang TY, et al. The Advent of Ultra-high Volume Thoracic Surgical Centers in Shanghai. World J Surg 2017;41:2758-68. [Crossref] [PubMed]
- Chong S, Lee KS, Chung MJ, et al. Lung cancer screening with low-dose helical CT in Korea: experiences at the Samsung Medical Center. Journal of Korean Medical Science 2005;20:402-8. [Crossref] [PubMed]
- Crucitti P, Gallo IF, Santoro G, et al. Lung cancer screening with low dose CT: experience at Campus Bio-Medico of Rome on 1500 patients. Minerva Chirurgica 2015;70:393-9. [PubMed]
- dos Santos RS, Franceschini JP, Chate RC, et al. Do Current Lung Cancer Screening Guidelines Apply for Populations With High Prevalence of Granulomatous Disease? Results From the First Brazilian Lung Cancer Screening Trial (BRELT1). The Annals of Thoracic Surgery 2016;101:481-6; discussion 487-8. [Crossref] [PubMed]
- Henschke CI, McCauley DI, Yankelevitz DF, et al. Early lung cancer action project: a summary of the findings on baseline screening. Oncologist 2001;6:147-52. [Crossref] [PubMed]
- International Early Lung Cancer Action Program Investigators. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. [Crossref] [PubMed]
- Lam VK, Miller M, Dowling L, et al. Community low-dose CT lung cancer screening: a prospective cohort study. Lung 2015;193:135-9. [Crossref] [PubMed]
- Luo X, Zheng S, Liu Q, et al. Should Nonsmokers Be Excluded from Early Lung Cancer Screening with Low-Dose Spiral Computed Tomography? Community-Based Practice in Shanghai. Transl Oncol 2017;10:485-90. [Crossref] [PubMed]
- McKee BJ, McKee AB, Flacke S, et al. Initial experience with a free, high-volume, low-dose CT lung cancer screening program. J Am Coll Radiol 2013;10:586-92. [Crossref] [PubMed]
- Menezes RJ, Roberts HC, Paul NS, et al. Lung cancer screening using low-dose computed tomography in at-risk individuals: the Toronto experience. Lung Cancer 2010;67:177-83. [Crossref] [PubMed]
- Nawa T, Nakagawa T, Kusano S, et al. Lung cancer screening using low-dose spiral CT: results of baseline and 1-year follow-up studies. Chest 2002;122:15-20. [Crossref] [PubMed]
- Nawa T, Nakagawa T, Mizoue T, et al. A decrease in lung cancer mortality following the introduction of low-dose chest CT screening in Hitachi, Japan. Lung Cancer 2012;78:225-8. [Crossref] [PubMed]
- Investigators NYELCAP. CT Screening for lung cancer: diagnoses resulting from the New York Early Lung Cancer Action Project. Radiology 2007;243:239-49. [Crossref] [PubMed]
- Sanchez-Salcedo P, Berto J, de-Torres JP, et al. Lung cancer screening: fourteen year experience of the Pamplona early detection program (P-IELCAP). Archivos De Bronconeumologia 2015;51:169-76. [PubMed]
- Swensen SJ, Jett JR, Hartman TE, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology 2003;226:756-61. [Crossref] [PubMed]
- Swensen SJ, Jett JR, Hartman TE, et al. CT screening for lung cancer: five-year prospective experience. Radiology 2005;235:259-65. [Crossref] [PubMed]
- Toyoda Y, Nakayama T, Kusunoki Y, et al. Sensitivity and specificity of lung cancer screening using chest low-dose computed tomography. Br J Cancer 2008;98:1602-7. [Crossref] [PubMed]
- Veronesi G, Bellomi M, Mulshine JL, et al. Lung cancer screening with low-dose computed tomography: a non-invasive diagnostic protocol for baseline lung nodules. Lung Cancer 2008;61:340-9. [Crossref] [PubMed]
- Veronesi G, Maisonneuve P, Spaggiari L, et al. Diagnostic performance of low-dose computed tomography screening for lung cancer over five years. J Thorac Oncol 2014;9:935-9. [Crossref] [PubMed]
- Ahmed A, Verma N, Barreto I, et al. Low-dose Lung Cancer Screening at an Academic Medical Center: Initial Experience and Dose Reduction Strategies. Acad Radiol 2018;25:1025-30. [Crossref] [PubMed]
- Blanchon T, Bréchot J-M, Grenier PA, et al. Baseline results of the Depiscan study: a French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR). Lung Cancer 2007;58:50-8. [Crossref] [PubMed]
- Gohagan J, Marcus P, Fagerstrom R, et al. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the Lung Screening Study of the National Cancer Institute. Chest 2004;126:114-21. [Crossref] [PubMed]
- Gohagan JK, Marcus PM, Fagerstrom RM, et al. Final results of the Lung Screening Study, a randomized feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer 2005;47:9-15. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Infante M, Cavuto S, Lutman FR, et al. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med 2009;180:445-53. [Crossref] [PubMed]
- Infante M, Cavuto S, Lutman FR, et al. Long-Term Follow-up Results of the DANTE Trial, a Randomized Study of Lung Cancer Screening with Spiral Computed Tomography. Am J Respir Crit Care Med 2015;191:1166-75. [Crossref] [PubMed]
- Pedersen JH, Ashraf H, Dirksen A, et al. The Danish randomized lung cancer CT screening trial--overall design and results of the prevalence round. J Thorac Oncol 2009;4:608-14. [Crossref] [PubMed]
- Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax 2012;67:296-301. [Crossref] [PubMed]
- Wille MM, Dirksen A, Ashraf H, et al. Results of the Randomized Danish Lung Cancer Screening Trial with Focus on High-Risk Profiling. Am J Respir Crit Care Med 2016;193:542-51. [Crossref] [PubMed]
- Lopes Pegna A, Picozzi G, Falaschi F, et al. Four-year results of low-dose CT screening and nodule management in the ITALUNG trial. J Thorac Oncol 2013;8:866-75. [Crossref] [PubMed]
- Paci E, Puliti D, Lopes Pegna A, et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 2017;72:825-31. [Crossref] [PubMed]
- Becker N, Motsch E, Gross ML, et al. Randomized study on early detection of lung cancer with MSCT in Germany: study design and results of the first screening round. J Cancer Res Clin Oncol 2012;138:1475-86. [Crossref] [PubMed]
- Becker N, Motsch E, Gross ML, et al. Randomized Study on Early Detection of Lung Cancer with MSCT in Germany: Results of the First 3 Years of Follow-up After Randomization. J Thorac Oncol 2015;10:890-6. [Crossref] [PubMed]
- Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 2012;21:308-15. [Crossref] [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [Crossref] [PubMed]
- Oudkerk M, Heuvelmans MA. Screening for lung cancer by imaging: the Nelson study. JBR-BTR 2013;96:163-6. [PubMed]
- Walter JE, Heuvelmans MA, de Jong PA, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncol 2016;17:907-16. [Crossref] [PubMed]
- Yousaf-Khan U, van der Aalst C, de Jong PA, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax 2017;72:48-56. [Crossref] [PubMed]
- Field JK, Duffy SW, Baldwin DR, et al. UK Lung Cancer RCT Pilot Screening Trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161-70. [Crossref] [PubMed]
- Yang W, Qian F, Teng J, et al. Community-based lung cancer screening with low-dose CT in China: Results of the baseline screening. Lung Cancer 2018;117:20-6. [Crossref] [PubMed]
- Cancer IAftSoL. NELSON Study Shows CT Screening for Nodule Volume Management Reduces Lung Cancer Mortality by 26 Percent in Men. 2018.
- Chen CY, Chen CH, Shen TC, et al. Lung cancer screening with low-dose computed tomography: Experiences from a tertiary hospital in Taiwan. J Formos Med Assoc 2016;115:163-70. [Crossref] [PubMed]
- Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:94S-107S.
- Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax 2013;68:551-64. [Crossref] [PubMed]
- Zhong C, Sakurai H, Wei S, et al. Sublobar resections for small-sized stage Ia lung adenocarcinoma: a Sino-Japanese multicenter study. J Thorac Dis 2018;10:991-8. [Crossref] [PubMed]
- Network NCC. NCCN Guidelines for Non-Small Cell Lung Cancer. 2017. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 29 April 2019.
- Patz EF Jr, Pinsky P, Gatsonis C, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014;174:269-74. [Crossref] [PubMed]
- Raz DJ, Zell JA, Ou SH, et al. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest 2007;132:193-9. [Crossref] [PubMed]
- Rosenberg RD, Yankaskas BC, Abraham LA, et al. Performance benchmarks for screening mammography. Radiology 2006;241:55-66. [Crossref] [PubMed]
- Society HKA-C. Lung Cancer. Available online: https://www.hkacs.org.hk/en/knowcancer_detail.php?id=3. Accessed 29 April 2019.
- Thun MJ, Henley SJ, Burns D, et al. Lung cancer death rates in lifelong nonsmokers. J Natl Cancer Inst 2006;98:691-9. [Crossref] [PubMed]
- Machiela MJ, Hsiung CA, Shu XO, et al. Genetic variants associated with longer telomere length are associated with increased lung cancer risk among never-smoking women in Asia: a report from the female lung cancer consortium in Asia. Int J Cancer 2015;137:311-9. [Crossref] [PubMed]
- Seow WJ, Matsuo K, Hsiung CA, et al. Association between GWAS-identified lung adenocarcinoma susceptibility loci and EGFR mutations in never-smoking Asian women, and comparison with findings from Western populations. Hum Mol Genet 2017;26:454-65. [PubMed]
- Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res 2008;8:165-78. [Crossref] [PubMed]
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796-7. [Crossref] [PubMed]
- Excellence NIfHaC. Guide to the processes of technology appraisal. 2018.
- Puggina A, Broumas A, Ricciardi W, et al. Cost-effectiveness of screening for lung cancer with low-dose computed tomography: a systematic literature review. Eur J Public Health 2016;26:168-75. [Crossref] [PubMed]
Cite this article as: Yu SWY, Leung CS, Tsz CH, Lee BTY, Chan HK, Sihoe ADL. Does low-dose computed tomography screening improve lung cancer-related outcomes?—a systematic review. Video-assist Thorac Surg 2020;5:7.