Uniportal video-assisted thoracic total pleural covering for refractory pneumothorax in a patient with lymphangioleiomyomatosis: a case report
Introduction
Uniportal video-assisted thoracic surgery (U-VATS) is a sophisticated and widespread method for various thoracic surgical procedures. The safety and efficacy of U-VATS compared to multi-portal VATS have been confirmed by multiple reports using propensity match analysis, randomized study, or systematic review and meta-analysis (1-3). A spontaneous pneumothorax is a major and frequently recurrent complication of lymphangioleiomyomatosis (LAM) because of widespread multiple lung cysts (4,5), which have been reported to be difficult to control by surgical resection of affected lungs with or without chemical or surgical pleurodesis (6). Recently, total pleural covering (TPC) via 4-port video-assisted thoracic surgery (4P-VATS) has been reported as a new surgical procedure that covers the entire surface of the lung with sheets of oxidized regenerated cellulose (ORC) and successfully reduces the recurrence rate of post-operative pneumothorax in LAM patients (7). These sheets reinforce the total pleura through pleural thickening (7,8). Initially, this procedure needs 4P-VATS; however, due to advances in the U-VATS technique in recent years, U-VATS TPC has become feasible. Herein we report a case of secondary pneumothorax in a patient with LAM who was successfully treated by U-VATS TPC with ORC mesh. Because this is the first case report of U-VATS TPC for secondary pneumothorax in a patient of LAM, we presented the following unique case in accordance with the CARE Guideline (9).
Case presentation
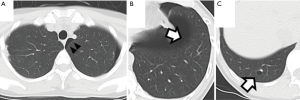
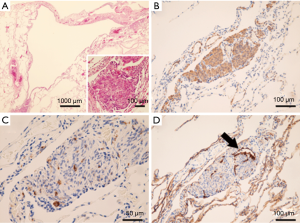
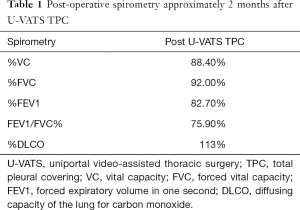
The patient was a 41-year-old woman with a left-sided spontaneous pneumothorax. The medical and family histories were unremarkable. She sought evaluation at a local outpatient clinic with a chief complaint of left-sided chest and back pain and dyspnea. A chest radiograph was consistent with the diagnosis of a left pneumothorax. Because of a refractory pneumothorax, she was referred to our department to undergo surgery. Laboratory testing revealed mild anemia. Computed tomography showed bilateral multiple cysts and a secondary spontaneous pneumothorax (Figure 1). Diseases causing multiple lung cysts and a secondary pneumothorax, such as Birt-Hogg-Dubé syndrome, LAM, and Langerhans cell histiocytosis, were in the differential diagnosis. We performed a U-VATS partial resection, ablation, and ligation of the affected lungs, followed by TPC for the left pneumothorax due to multiple lung cysts under general anesthesia with one lung ventilation. Briefly, for U-VATS TPC, a 5-cm incision on the middle axillary line of the 6th intercostal region was made and a wound retractor (Alexis® Wound Protector/Retractor S; Applied Medical, Rancho Santa Margarita, CA, USA) was applied for the single port. TPC consisted of completely enclosing the entire surface of the fragile lungs with multiple lung cysts on the surgical side with 12 sheets of ORC mesh (Ethicon Surgical absorbable hemostat gauze; Johnson & Johnson, Brunswick, NJ, USA), followed by 6 milliliter drops of fibrin glue (Beriplast® P combi-set tissue adhesion; CSL Behring, King of Prussia, PA, USA). To complete the TPC procedure, a 20-Fr drainage tube was placed into the apex of the thoracic cavity. Close inspection confirmed that the ORC-covered lungs were fully expanded (Figure 2), as reported previously (11). The chest drainage tube was removed on the 4th post-operative day. The patient was discharged from the hospital on the 7th post-operative day. Histologic examination of the resected specimen showed multiple lung cysts, including LAM cells (Figure 3A,B,C,D), resulting in the diagnosis of sporadic LAM. The post-operative course was uneventful. Approximately 2 months after the TPC, a chest radiograph showed no recurrence of the pneumothorax with good expansion of the left covered lungs (Figure 4). A post-operative spirogram showed a normal vital capacity (VC), forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and diffusing capacity of the lung for carbon monoxide (DLCO; Table 1). While the timeline of the case was shown in Figure 5, the adhesion and tolerability of the U-VATS TPC seemed to be feasible because the intervention was minimally invasive and the outcome was outstanding without adverse and unanticipated events.


Full table
Discussion
We successfully performed U-VATS TPC without surgical or post-operative complications. U-VATS potentially damages only one intercostal region, has been reported to minimize post-operative thoracic pain, leads to a cosmetic advantage (3), and the surgical indication is expanding among general thoracic procedures (12). The patient in this case report did not have epidural anesthesia, had only one incision, and tolerated minimal post-operative pain. In contrast, post-operative discomfort due to the chest drainage tube existed; however, the discomfort resolved after removal of the tube. On the 7th and 30th post-operative days, the patient had no pain or discomfort with no analgesics.
LAM is a rare disease that affects between 5 and 9 million women of childbearing age. LAM is known as a disease that affects the lungs, resulting in multiple lung cysts, recurrent pneumothoraces, and dyspnea. The lymphatics are also affected, leading to lymphatic masses and chylous collections. In addition, LAM leads to angiomyolipomas, a mixed mesenchymal tumor in the kidneys. The natural history of LAM is highly variable, but most women with LAM are prone to pneumothoraces, and recurrent pneumothoraces lead to a loss of lung function at an accelerated rate, development of progressive dyspnea, and even respiratory failure and death (13).
A standard surgical approach, such as resection of the affected lung, cannot control the inevitable recurrence because multiple fragile cysts that may occur with a pneumothorax appear diffusely on the surface of the visceral pleura. Owing to the morbidity and excessive costs associated with multiple recurrences in these patients, early intervention with chemical and surgical pleurodesis has been performed. Severe pleural adhesions caused by chemical and surgical pleurodesis administered to such patients who later undergo lung transplantation frequently make the procedures technically difficult, and lead to life-threatening bleeding (6).
Ebana et al. (8) reported that an ORC mesh covering causes pleural thickening via the mesothelial-mesenchymal transition (8). The Gynecare Interceed Absorbable Adhesion Barrier (Johnson & Johnson, Brunswick, NJ, USA), which is composed of the same ORC as the ORC mesh, is indicated as an adjunct to gynecologic pelvic surgery for reducing the incidence of post-operative pelvic adhesions. Kurihara et al. (7) reported that 4P-VATS TPC with ORC mesh reduced the recurrence rate and frequency of post-operative pneumothoraces in patients with LAM and partial adhesions of the thoracic cavity (7), which may facilitate future surgery, such as lung transplantation. The patient shared her perspective on the various complications attributed to LAM, and both the advantage and disadvantage of the several treatments for the fragile lungs.
Conclusions
We report a case of a secondary pneumothorax in a patient of LAM who was successfully treated by TPC with ORC mesh via U-VATS which seems to be a unique strategy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/vats.2019.12.01). Teruaki Mizobuchi serves as an unpaid editorial board member of Video-Assisted Thoracic Surgery from Aug 2019 to Jul 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The patient shared her perspective comprehensively. Written informed consent was obtained from the patient for the case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nachira D, Ismail M, Meacci E, et al. Uniportal vs. triportal video-assisted thoracic surgery in the treatment of primary pneumothorax-a propensity matched bicentric study. J Thorac Dis 2018;10:S3712-9. [Crossref] [PubMed]
- Kutluk AC, Kocaturk CI, Akin H, et al. Which is the Best Minimal Invasive Approach for the Treatment of Spontaneous Pneumothorax? Uniport, Two, or Three Ports: A Prospective Randomized Trail. Thorac Cardiovasc Surg 2018;66:589-94. [Crossref] [PubMed]
- Yang W, Zhang G, Pan S, et al. Comparison of the perioperative efficacy between single-port and two-port video-assisted thoracoscopic surgery anatomical lung resection for non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 2019;11:2763-73. [Crossref] [PubMed]
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008;133:507-16. [Crossref] [PubMed]
- Seyama K, Kumasaka T, Kurihara M, et al. Lymphangioleiomyomatosis: a disease involving the lymphatic system. Lymphat Res Biol 2010;8:21-31. [Crossref] [PubMed]
- Almoosa KF, Ryu JH, Mendez J, et al. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest 2006;129:1274-81. [Crossref] [PubMed]
- Kurihara M, Mizobuchi T, Kataoka H, et al. A Total Pleural Covering for Lymphangioleiomyomatosis Prevents Pneumothorax Recurrence. PLoS One 2016;11:e0163637 [Crossref] [PubMed]
- Ebana H, Hayashi T, Mitani K, et al. Oxidized regenerated cellulose induces pleural thickening in patients with pneumothorax: possible involvement of the mesothelial-mesenchymal transition. Surg Today 2018;48:462-72. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Oshima K, Mizobuchi T, Nagato K, et al. Total pleural covering (TPC) procedure of the left lungs using uniportal video-assisted thoracic surgery (U-VATS) in a patient with lymphangioleiomyomatosis. Asvide 2020;7:053. Available online: http://www.asvide.com/watch/33093
- Mizobuchi T, Kurihara M, Ebana H, et al. A total pleural covering of absorbable cellulose mesh prevents pneumothorax recurrence in patients with Birt-Hogg-Dubé syndrome. Orphanet J Rare Dis 2018;13:78. [Crossref] [PubMed]
- Guido-Guerrero W, Bolaños-Cubillo A, González-Rivas D. Single-port video-assisted thoracic surgery (VATS)-advanced procedures & update. J Thorac Dis 2018;10:S1652-61. [Crossref] [PubMed]
- Johnson SR, Taveira-DaSilva AM, Moss J. Lymphangioleiomyomatosis. Clin Chest Med 2016;37:389-403. [Crossref] [PubMed]
Cite this article as: Oshima K, Mizobuchi T, Nagato K, Ishibashi F, Sugano I, Kumasaka T. Uniportal video-assisted thoracic total pleural covering for refractory pneumothorax in a patient with lymphangioleiomyomatosis: a case report. Video-assist Thorac Surg 2020;5:10.